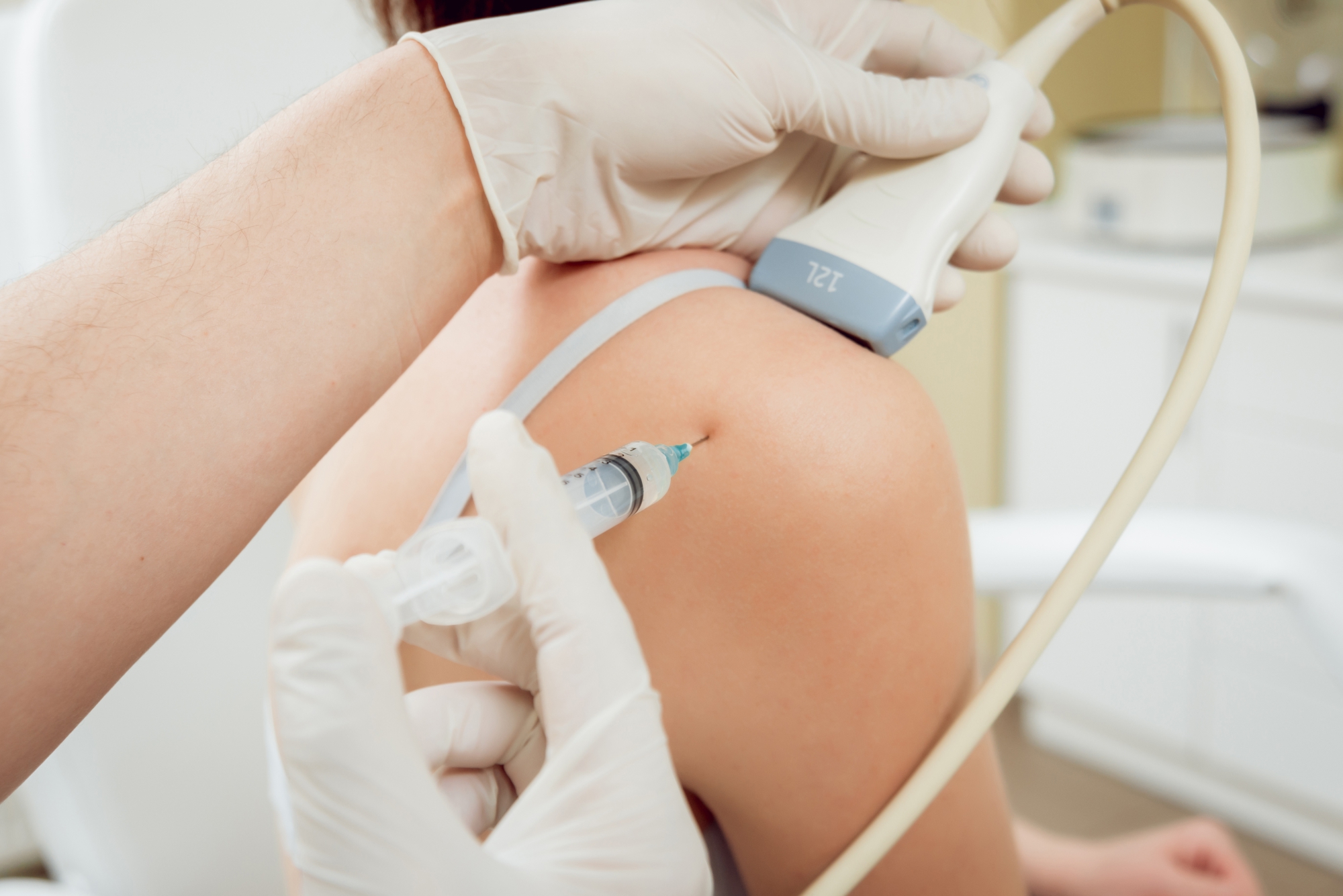
Hyaluronic Acid (HA) Injection Versus Control for Painful Shoulder Conditions: A Systematic Review and Meta-Analysis of Randomized Controlled Trials .
Shoulder pain affects millions worldwide and is a leading cause of disability, particularly among older adults. Evidence from 13 randomized controlled trials comparing hyaluronic acid (HA) injections with control treatments for painful shoulder conditions was analyzed. Low to moderate quality evidence showed that HA provides small but measurable pain improvements at 3 and 6 months, and modest functional gains at 3 months, though effects did not reach minimal important difference thresholds. No benefits were found at 1 month, and functional improvement was not sustained at 6 months. HA was well tolerated, with only mild post-injection reactions reported and no significant differences in adverse or serious adverse events. Two ongoing clinical trials are expected to add new data.
Unlock the Full original article
You have access to 4 more FREE articles this month.
Click below to unlock and view this original article
Unlock Now
Critical appraisals of the latest, high-impact randomized controlled trials and systematic reviews in orthopaedics
Access to OrthoEvidence podcast content, including collaborations with the Journal of Bone and Joint Surgery, interviews with internationally recognized surgeons, and roundtable discussions on orthopaedic news and topics
Subscription to The Pulse, a twice-weekly evidence-based newsletter designed to help you make better clinical decisions
Exclusive access to original content articles, including in-house systematic reviews, and articles on health research methods and hot orthopaedic topics
Or upgrade today and gain access to all OrthoEvidencecontent for as little as $1.99 per week.
Already have an account? Log in
Are you affiliated with one of our partner associations?
Click here to gain complimentary access as part your association member benefits!