Knee osteoarthritis (OA) affects about 1 in 6 people who are 15 years or older (Cui et al., 2020). Among people who are 70 years or older, the prevalence is as high as 40% (Hsu et al., 2020). Knee OA is the most common cause of chronic pain and often associated with impared function in the knee (Zhang et al., 2010).
Intra-articular (IA) injection of corticosteroids is widely used and conditionally recommended for patients with knee OA, with the aim of suppressing the inflammatory response in the joint. However, the effect of corticosteroid injection in pain relief is usually short-term, in most cases lasting 6 weeks (Bannuru et al., 2019; Johal et al., 2019).
Platelet-rich plasma (PRP) is a biological approach that has gained increasing attention in medical research and practice over the past 3 decades (Foster et al., 2009). PRP contains bioactive proteins (e.g., adhesion proteins, cytokines, chemokines and coagulation proteins) and growth factors that could promote healing and recovery of degenerative conditions. For patients with knee OA, PRP is infiltrated into the local anatomical region through a minimally invasive IA injection. As a result, patients may experience improvement in pain and function (Ayhan et al., 2014; Bannuru et al., 2019). Several available systematic reviews reported that PRP was a safe and effective treatment for knee OA (McLarnon et al., 2021; Nie et al., 2021). However, a new trial (Elksninš-Finogejevs et al., 2020) has not been included in these published systematic reviews.
In this OE Original, we conducted a systematic review and meta-analysis of randomized controlled trials (RCTs) and examined the most up-to-date evidence comparing IA injection of PRP versus corticosteroids in patients with knee OA.
Methods
We searched Ovid MEDLINE, Ovid EMBASE, Cochrane Controlled Register of Trials (CENTRAL), and OrthoEvidence from database inception to October 30, 2022 with both indexed terms and free text terms regarding PRP and knee OA (or chronic knee pain). Reference lists and existing systematic reviews were also searched to identify additional eligible studies.
Studies were eligible for inclusion if they met the following criteria: RCTs that compared PRP injection with corticosteroid injection for adult patients who were diagnosed with knee OA, and that were published in English with full texts available. Conference abstracts were excluded.
We are presenting the meta-analysis results for pain, function, and safety outcomes. For dichotomous outcomes, we presented risk ratios (RRs) and 95% confidence intervals (CIs). For continuous outcomes, we presented the weighted mean difference (MD) and 95% CI. For studies with outcomes for more than two eligible treatment or control arms — for example, if a study has one treatment arm and two control arms — we divided the data in the treatment arm by 2 in order to avoid double counting for studies with multiple arms (Higgins et al., 2022). We presented subgroup analysis results by dose of PRP (single or multiple injections). We rated the quality of evidence by GRADE assessment and applied the recommended minimal clinically important difference (MCID) for the respective outcomes to assess the magnitude of effects.
Results
1. Characteristics of included studies
We identified 10 RCTs investigating the effectiveness of the PRP IA injection with corticosteroids IA injection for pain and/or function improvement in patients with knee OA (Table 1). The majority of included studies were conducted in Asia (N=6, 60%), followed by Europe (N=2, 20%), Africa (N=1) and South America (N=1). One study was supported by the government (Joshi Jubert et al., 2017); two studies received institutional funding (Forogh et al., 2016; Nabi et al., 2018); three studies received no financial support (Elksninš-Finogejevs et al., 2020; Ismaiel et al., 2018; Uslu Guvendi et al., 2018); and four studies did not provide relevant information on funding (Freire et al., 2018; Huang et al., 2019; Khan et al., 2018; Phul et al., 2018).
A single injection of PRP was used in 8 studies, and multiple injections were used in 3 studies (two PRP injections in 1 study, three injections in 2 studies (Table 1). Of these, one study had three relevant arms including a single injection arm and a three-injection arm (Uslu Guvendi et al., 2018). Patient-reported outcomes were available at 1 month for five studies (Elksninš-Finogejevs et al., 2020; Freire et al., 2018; Ismaiel et al., 2018; Joshi Jubert et al., 2017; Nabi et al., 2018), at 2 months for three studies (Forogh et al., 2016; Nabi et al., 2018; Uslu Guvendi et al., 2018), at 3 months for five studies, at 6 months for ten studies, and at 12 months for two studies (Figures 2, 3). The characteristics of the RCTs and the study arms included in meta-analysis are presented in Table 1.
Table 1. Characteristics of RCTs included in meta-analysis
Author, Year | Country | Number of patients/knees (K-L grade) | Age (years)* | Sex (F/M) | IA Injection of PRP | IA Injection of Corticosteroid** |
Elksninš-Finogejevs et al., 2020 | Latvia | 40 | PRP: 66.4 (8.4) CS: 70.2 (9.2) | 8/32 | Single injection; 8 mL of autologous pure PRP; Ultrasound-guided (echographic control). | A mixture of 1 mL of 40 mg/mL triamcinolone acetonide and 5 mL of 2% lidocaine; Ultrasound-guided. |
Forogh et al., 2016 | Iran | 48 knees in 41 patients (Grade 2/3: 15/33) | PRP: 59.13 (7.03) CS: 61.13 (6.7) | 32/16 | Single injection; 5 mL of autologous leukocyte rich-PRP. | 1 mL of 40 mg of methylprednisolone acetate. |
Freire et al., 2018 | Brazil | 50 (Grade 1/2/3/4: 1/20/24/4) | PRP: 64.15 (8.02) CS: 60.21 (5.92) | 42/8 | Single injection; 5 mL of autologous leukocyte poor-PRP. | 2.5 mL of 20 mg/mL triamcinolone acetate. |
Huang et al., 2019 | China | 80 (Grade 1-2; no. NR) | PRP: 54.5 (1.2) CS: 54.3 (1.4) | 34/46 | Single injection; 4 mL of autologous leukocyte poor-PRP. | 1 mL of a corticosteroid, type not specified. |
Ismaiel et al., 2018 | Egypt | 60/92 (Grade 3/4: 54/38 | PRP: 62.9 (11.6) CS: 61.1 (11.6) | 60/32 | Single injection; 4–6 mL of autologous leukocyte rich-PRP. | Single injection of a corticosteroid, type and dose not specified. |
Joshi Jubert et al., 2017 | Spain | 65 (Grade 3/4: 27/38 | PRP: 65.56 (8.6) CS: 68 (7.17) | 47/18 | Single injection; 4 mL of autologous leukocyte poor-PRP. | A mixture of 6 mg betamethasone sodium phosphate and betamethasone acetate, and 2 mL of 0.25% bupivacaine. |
Khan et al., 2018 | Pakistan | 102 (all were Grade 2) | PRP: 50.91 (13.07) CS: 52.09 (12.1) | 77/25 | Two injections given 2 months apart; 5 mL of PRP at each injection. | A mixture of 1 mL of 40 mg/mL triamcinolone acetonide and 4 mL of 1% lidocaine hydrochloride. |
Nabi et al., 2018 | Iran | 67 (Grade 2/3: 20/47) | PRP: 59.09 (7.79) CS: 58.55 (8.79) | 55/12 | Three injections given once a month; 5 mL leukocyte rich-PRP; Ultrasound-guided. | 40 mg triamcinolone; Ultrasound-guided. |
Phul et al., 2018 | Pakistan | 80 (Grade 2-4; no. NR) | PRP: 54.45 (4.54) CS: 57.65 (10.36) | 54/26 | Single injection; 4–6 µmL of autologous leukocyte rich- PRP. | 4 mL of 40 mg/mL triamcinolone hexacetonide and 10 mg bupivacaine; fluoroscopically guided. |
Uslu Guvendi et al., 2018 | Turkey | 57 (all were Grade 3) | 61.3 (6.7) | 50/7 | a) Single injection; autologous leukocyte-rich PRP, dose not specified. b) Three injections given 1 week apart; autologous leukocyte-rich PRP. | Single injection; 1 mL of suspension containing 6.43 mg betamethasone dipropionate and 2.63 mg betamethasone sodium phosphate. |
Notes: K-L grade, Kellgren-Lawrence grading scale; F, female; M, male; IA, intra-articular; PRP, platelet-rich plasma; CS, corticosteroid.
Huang et al., 2019 is a 3-arm RCT that compares injections of PRP, corticosteroid, and hyaluronic acid. Uslu Guvendi et al., 2018 is a 3-arm RCT that compares single injection of PRP, 3 injections of PRP, and single injection of a corticosteroid.
* Age was presented as mean and standard deviation (SD) unless otherwise specified.
** Same frequencies of injection and procedures in the corticosteroid group as the PRP group unless otherwise specified.
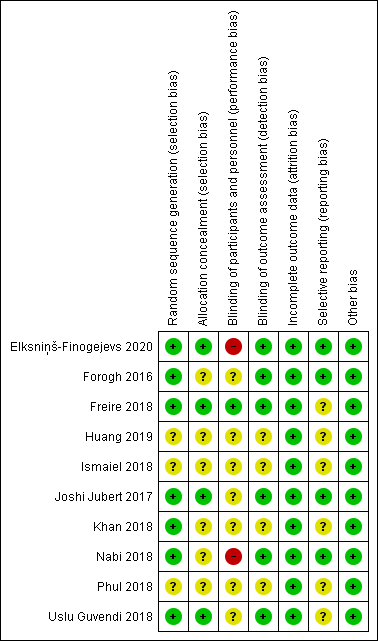
In terms of risk of bias (Figure 1), three to six RCTs did not provide adequate information in random sequence generation, allocation concealment, blinding of outcome assessment, and selective reporting. Except for one RCT (Freire et al., 2018), all were rated as having high or unclear risk of bias in blinding of participants and treatment providers. All of the included RCTs had a low risk of bias in incomplete outcome data.
Figure 1. Risk of bias assessment
2. Quantitative synthesis
We are presenting the meta-analysis results of pain and function at four follow-up time points: 1-2 months, 3 months, 6 months, and 12 months post intervention as well as incidence of adverse events at the longest follow-up period.
2.1 Pain score (0 to 10, a higher score indicates worse pain)
The included studies reported visual analogue scale (VAS) pain on a 0-10 or 0-100 scale at several follow-up time points. We normalized the scores on a 0 to 10 scale and utilized the MCID of 1.0 to assess pain outcome in patients with knee OA (Concoff et al., 2019).
In the comparison of PRP injection versus corticosteroid injection for pain, the point estimates of overall effect favours PRP at all the follow-up time points. At the shortest follow-up of 1 to 2 months (348 patients from 6 studies; MD, -0.98; 95% CI, -2.10 to 0.13), and the longest follow-up of 12 months (116 patients from 2 studies; MD, -1.19; 95% CI, -3.06 to 0.69), there were no significant differences between the two groups with the upper boundaries of the 95% CIs having crossed the no-effect threshold of 0. The certainty of the evidence was rated as very low due to risk of bias, imprecision and inconsistency (Figure 2).
At 3 months, the meta-analysis of a total of 259 patients from 4 studies published between 2017 and 2020 demonstrates that PRP results in a significant improvement with patients experiencing, on average, a 0.83 (95% CI, 0.14 to 1.51) point less pain than patients who received corticosteroids injection. The effect and 95% CI did not exceed the MCID of 1.0 point on the 0 to 10 scale. The certainty of the evidence was rated as low due to risk of bias, imprecision and inconsistency (Figure 2).
At 6 months of follow-up, a total of 530 patients from 8 studies published between 2016 and 2020 were included in analysis. The overall effect demonstrates that PRP results in a significant improvement with patients experiencing, on average, a 1.59 (95% CI, 0.77 to 2.40) point less pain than patients in the corticosteroids group. The effect and 95% CI did not exceed the MCID of 1.0 point. The certainty of the evidence was rated as very low (Figure 2).
Figure 2. Forest plot of pain on a 0-10 scale
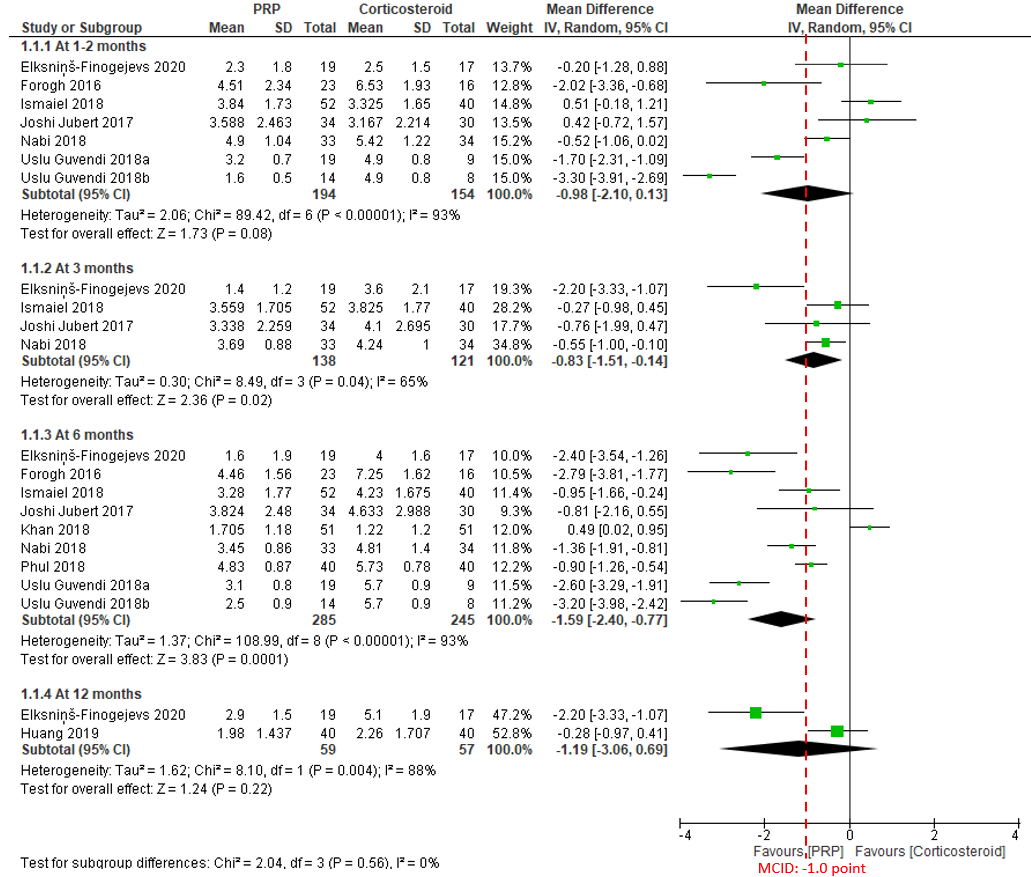
Notes: PRP = platelet-rich plasma; MCID = minimal clinically important difference.
2.2 Function (0 to 100, a higher score indicates better recovery)
Knee injury and Osteoarthritis Outcome Score (KOOS) subscales, Knee Society Clinical Rating System (KSS), the Western Ontario and McMaster Universities Arthritis Index (WOMAC) total and subscale scores were normalized on a 0 to 100 scale to assess patient function (Velentgas et al., 2013).
The MCID of KOOS ADL (activities of daily living) score in patients with KOOS is found to be 17 points on the 0 to 100 scale (Jacquet et al., 2021).
In the current comparison of PRP versus corticosteroid injections for function at 1 to 2 months, a total of 398 patients from 7 studies published between 2016 and 2020 were included in the analysis. The overall effect demonstrates that PRP results in a significant improvement with patients experiencing, on average, a 4.11 (95% CI, 1.08 to 7.13) point improvement. The effect and 95% CI did not exceed the recommended MCID of 17 points on the 0 to 100 scale. The certainty of the evidence was rated as low due to risk of bias and inconsistency (Figure 3).
At 3 months of follow-up, the overall effect of 339 patients from 5 studies shows that there is no significant difference between PRP and corticosteroid, with very low certainty of evidence due to risk of bias, imprecision and inconsistency (Figure 3).
At 6 months of follow-up, a total of 580 patients from 9 studies published between 2016 and 2020 were included in analysis. The overall effect demonstrates that PRP results in a significant improvement with patients experiencing, on average, a 10.57 (95% CI, 6.39 to 14.76) point improvement. The effect and 95% CI did not exceed the MCID of 17 points. The certainty of the evidence was rated as low due to risk of bias and inconsistency (Figure 3).
At 12 months of follow-up, the overall effect of 116 patients from 2 studies demonstrates that PRP results in a significant improvement with patients experiencing, on average, a 16.78 (95% CI, 13.77 to 19.79) point improvement. The effect and 95% CI did not exceed the MCID of 17 points. The certainty of the evidence was rated as low due to risk of bias and imprecision (Figure 3).
Figure 3. Forest plot of function on a 0-100 scale
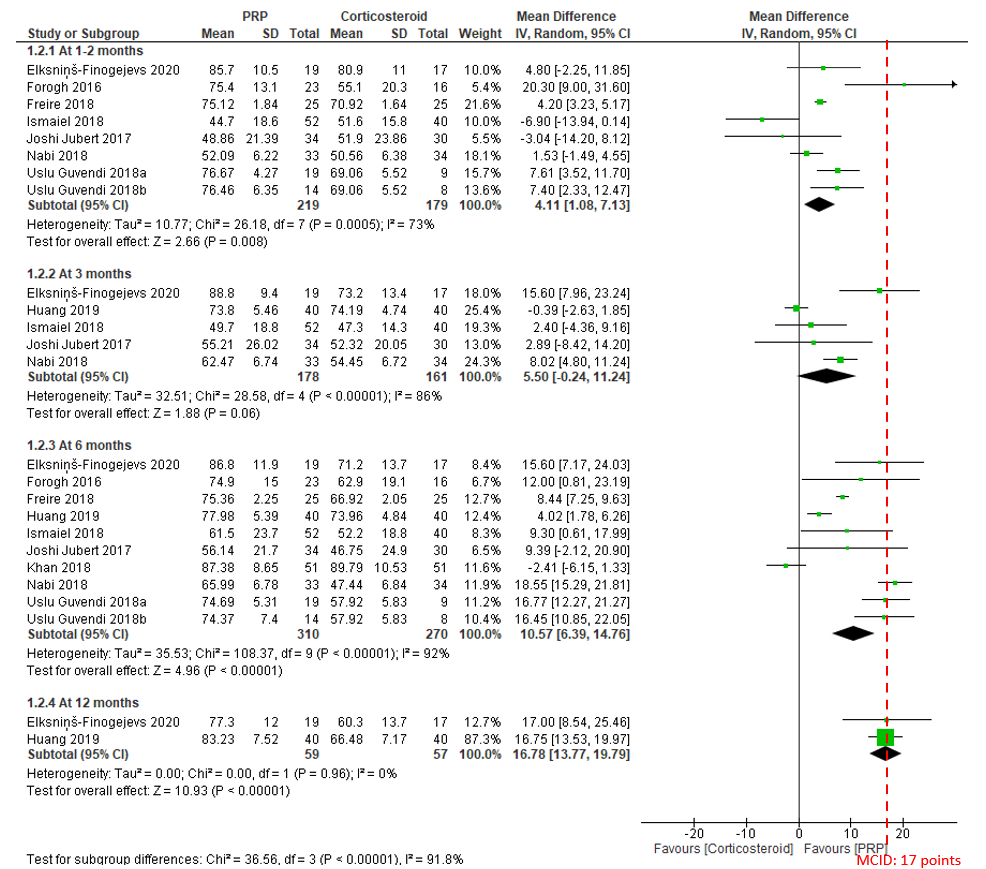
Notes: PRP = platelet-rich plasma; MCID = minimal clinically important difference.
2.3 Incidence of adverse events
There was no significant difference in adverse events between PRP and corticosteroid at the longest follow-up (301 patients from 5 studies published between 2017 and 2020; RR, 3.77; 95% CI, 0.46 to 31.30), with low certainty of evidence (Figure 4). No serious adverse events were reported.
The reported adverse events included mild synovitis or mild erythema in the PRP group (Elksninš-Finogejevs et al., 2020; Uslu Guvendi et al., 2018), and mild to moderate pain in the injection site (Huang et al., 2019; Nabi et al., 2018). All these adverse events were resolved quickly.
Figure 4. Forest plot of adverse events
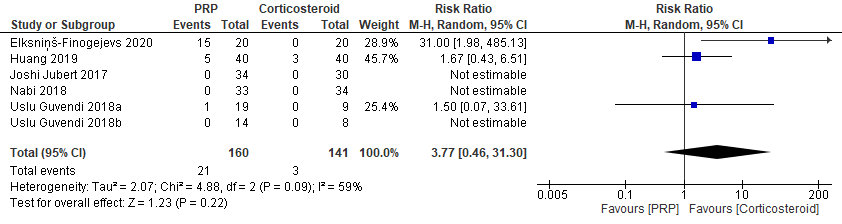
Notes: PRP = platelet-rich plasma.
We present a summary of the four outcome measures at five follow-up time points in Table 2.
Table 2. Summary and certainty of the evidence
Outcome | 1 to 2 months | 3 months | 6 months | 12 months |
Pain | No difference (Very low certainty) | Favours PRP (Very low certainty) | Favours PRP (Very low certainty) | No difference (Very low certainty) |
Function | Favours PRP (Low certainty) | No difference (Very low certainty) | Favours PRP (Low certainty) | Favours PRP (Low certainty) |
Adverse events | No difference (Low certainty) | |||
Notes: PRP, platelet-rich plasma. The effect and 95% confidence interval did not exceed the recommended minimal clinically important difference for pain or function outcomes at all the follow-up time points.
2.4 Subgroup analysis by dose of PRP
We presented the subgroup analysis by PRP dose (single injection or multiple injections) in Table 3. We did not find any subgroup differences/effects for pain, function or adverse events at all follow-up time points (P values in test for subgroup difference > 0.05).
Table 3. Results of subgroup analysis by PRP dose
Outcome | Single dose of PRP vs. Corticosteroid (95% CI) | Multiple doses of PRP vs. Corticosteroid (95% CI)* | Test for Subgroup Difference (P value) |
Pain (1 to 2 months) | MD: -0.58 (-1.69 to 0.53) Based on data from 259 patients in 5 studies | MD: -1.91 (-4.63 to 0.82) Based on data from 89 patients in 2 studies | No significant difference (P = 0.38) |
Pain (3 months) | MD: -1.03 (-2.20 to 0.14) Based on data from 192 patients in 3 studies | MD: -0.55 (-1.00 to -0.10) Based on data from 67 patients in 1 study | No significant difference (P = 0.46) |
Pain (6 months) | MD: -1.72 (-2.51 to -0.94) Based on data from 339 patients in 6 studies | MD: -1.34 (-3.33 to 0.65) Based on data from 191 patients in 3 studies | No significant difference (P = 0.72) |
Pain (12 months) | MD: -1.19 (-3.06 to 0.69) Based on data from 116 patients in 2 studies | No data | Not applicable |
Function (1 to 2 months) | MD: 4.12 (-0.38 to 8.63) Based on data from 309 patients in 6 studies | MD: 4.10 (-1.61 to 9.80) Based on data from 89 patients in 2 studies | No significant difference (P = 0.99) |
Function (3 months) | MD: 4.78 (-2.43 to 11.98) Based on data from 272 patients in 4 studies | MD: 8.02 (4.80 to 11.24) Based on data from 67 patients in 1 study | No significant difference (P = 0.42) |
Function (6 months) | MD: 10.13 (6.46 to 13.80) Based on data from 389 patients in 7 studies | MD: 10.83 (-3.39 to 25.04) Based on data from 191 patients in 3 studies | No significant difference (P = 0.93) |
Function (12 months) | MD: 16.78 (13.77 to 19.79) Based on data from 116 patients in 2 studies | No data | Not applicable |
Adverse events | RR: 3.77 (0.46 to 31.30) Based on data from 212 patients in 4 studies | Not estimable (number of events = 0) | Not applicable |
Notes: PRP, plasma-rich platelet; CI, confidence interval; MD, mean difference. Pain (scale of 0 to 10, a higher score indicates worse pain); Function (scale of 0 to 100, a higher score indicates better function).
* Multiple doses of PRP in the included RCTs were: two injections given 2 months apart, and three injections given 1 week apart or 1 month apart (details can be found in Table 1).
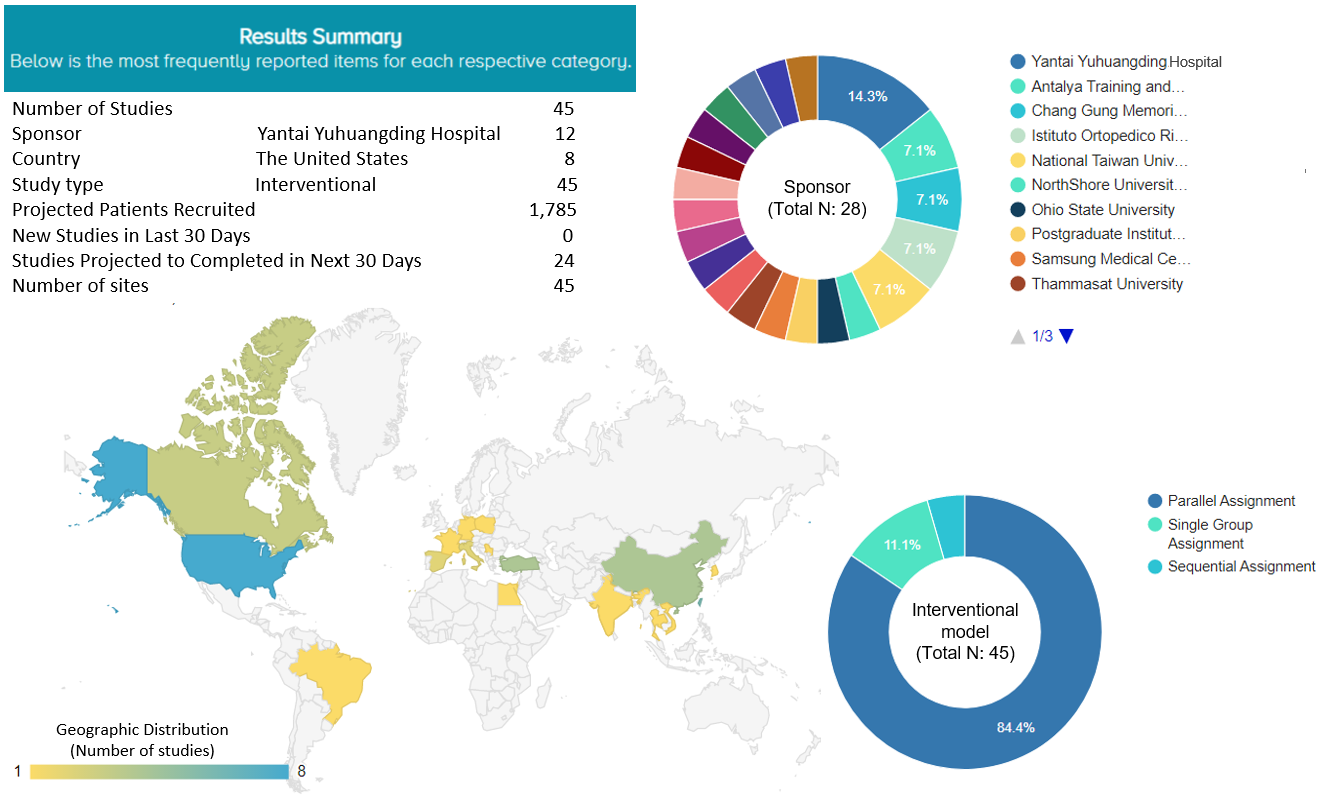
3. OE M.I.N.D. Ongoing trials report
We found 45 registered, ongoing studies that are investigating the effects of PRP or corticosteroid injection in treating knee OA. They are all interventional studies aiming to recruit 1,785 patients. Eight of these ongoing studies (17.8%) are being conducted in the United States. The top sponsors are from Asian countries or regions, namely, Yantai Yuhuangding Hospital in China (N=4, 14.3%), two hospitals in Taiwan (Chang Gung Memorial Hospital, 2 studies, 7.1%; National Taiwan University, 2 studies, 7.1%), Antalya Education and Research Hospital in Turkey (N=2, 7.1%), and the Rizzoli Orthopedic Institute in Italy (N=2, 7.1%). The majority of the ongoing studies are parallel trials (N=38, 84.4%) (Figure 5).
Figure 5. Ongoing trials of PRP or corticosteroid injection for knee osteoarthritis
Discussion
In this OE Original, we identified 10 RCTs that investigated the efficacy and safety of PRP injection versus corticosteroid injection for patients with knee OA. In our meta-analysis, very low to low quality of evidence showed that PRP IA injection was associated with small effects compared to corticosteroid IA injection in pain improvement at 3 and 6 months of follow-up and in function outcomes at 1 to 2, 6 and 12 months of follow-up. The between-group differences were larger at longer follow-up time, for example, 6 and 12 months post-intervention for both pain and function (Figures 2, 3). For effects that were statistically significant, the 95% CI of pain and function did not exceed the recommended minimal clinically important difference. No difference was found for pain at 1 to 2 months and at 12 months, or for function at 3 months of follow-up.
There was no difference between PRP and corticosteroid injection in incidence of adverse events at the longest follow-up. No serious adverse events were reported after either PRP or corticosteroid injection in all the included RCTs.
The findings in this OE Original are consistent with previously published systematic reviews on the similar topic that PRP injection demonstrates effects in pain and OA symptom relief (McLarnon et al., 2021; Nie et al., 2021).
The results of subgroup analysis by the PRP dose showed that multiple doses had slightly larger between-group differences (greater effects) in the point estimates (the central MD values) for pain at 1 to 2 months of follow-up, and for function at 3 and 6 months of follow-up. On the contrary, a single dose of PRP injection showed slightly larger differences in the point estimates for pain at 3 and 6 months of follow-up, and for function at 1 to 2 months of follow-up. However, we were unable to draw any conclusions on subgroup effects in terms of single- versus multiple-dose PRP injection compared to corticosteroids injection because no significant differences were detected in tests for subgroup differences for any outcomes (Table 3). No studies investigating multiple doses of PRP reported outcomes at 12 months of follow-up; therefore, we were unable to evaluate the subgroup effects 12 months after intervention.
One of the major concerns in the evidence quality assessment was serious risk of bias due to lack of blinding to treatment providers and participants, as the necessary procedure of blood collection from patients in the PRP group posed an ethical challenge for patients in the corticosteroid group in most of the included studies (Guyatt et al., 2011a; Higgins et al., 2019). Six of the ten included studies reported blinding of outcome assessors. Because the patients were not blinded to the treatment that they received, there was a possibility of overestimation in the detected effects of the PRP injection. There were no missing outcome data in the current meta-analysis. Other major concerns were imprecision and inconsistency. We rated down one level of GRADE assessment for imprecision regarding the outcomes with no statistical difference, as well as for the outcomes showing statistical difference but not having the boundaries of 95% CIs extended the MCID values (Guyatt et al., 2011b). We rated down one level of GRADE quality of assessment for inconsistency for outcomes when the values of I^2 were greater than 40% (Guyatt et al., 2011c).
Additional future research with larger sample sizes and longer follow-up (e.g., longer than 12 months) is needed to comprehensively evaluate the outcomes, and verify the findings of the current meta-analysis results.
Bottom line
Meta-analysis of 10 RCTs showed that for patients with knee OA, PRP injection is associated with small benefits in pain and function compared to corticosteroids injection at various follow-up time points. There was no statistically significant difference in the incidence of adverse events between the PRP and corticosteroid groups. No serious adverse events were reported after PRP or corticosteroid IA injection.
Reference
Ayhan E, Kesmezacar H, Akgun I. Intraarticular injections (corticosteroid, hyaluronic acid, platelet rich plasma) for the knee osteoarthritis. World journal of orthopedics. 2014 Jul 7;5(3):351.
Bannuru RR, Osani MC, Vaysbrot EE, Arden NK, Bennell K, Bierma-Zeinstra SM, Kraus VB, Lohmander LS, Abbott JH, Bhandari M, Blanco FJ. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis and cartilage. 2019 Nov 1;27(11):1578-89.
Concoff A, Rosen J, Fu F, Bhandari M, Boyer K, Karlsson J, Einhorn TA, Schemitsch E. A comparison of treatment effects for nonsurgical therapies and the minimum clinically important difference in knee osteoarthritis: a systematic review. JBJS reviews. 2019 Aug;7(8):e5.
Cui A, Li H, Wang D, Zhong J, Chen Y, Lu H. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine. 2020 Dec 1;29:100587.
Elksninš-Finogejevs A, Vidal L, Peredistijs A. Intra-articular platelet-rich plasma vs corticosteroids in the treatment of moderate knee osteoarthritis: a single-center prospective randomized controlled study with a 1-year follow up. Journal of Orthopaedic Surgery and Research. 2020 Dec;15(1):257.
Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB, Rodeo SA. Platelet-rich plasma: from basic science to clinical applications. The American journal of sports medicine. 2009 Nov;37(11):2259-72.
Freire MR, da Silva PM, Azevedo AR, Silva DS, da Silva RB, Cardoso JC. Comparative effect between infiltration of platelet-rich plasma and the use of corticosteroids in the treatment of knee osteoarthritis: a prospective and randomized clinical trial. Revista Brasileira de Ortopedia. 2020 Dec 2;55:551-6.
Güvendi EU, Askin A, Güvendi G, Koçyigit H. Comparison of efficiency between corticosteroid and platelet rich plasma injection therapies in patients with knee osteoarthritis. Archives of Rheumatology. 2018 Sep;33(3):273.
Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P, Montori V, Akl EA, Djulbegovic B, Falck-Ytter Y, Norris SL. GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias). Journal of clinical epidemiology. 2011 Apr 1;64(4):407-15.
Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, Devereaux PJ, Montori VM, Freyschuss B, Vist G, Jaeschke R. GRADE guidelines 6. Rating the quality of evidence—imprecision. Journal of clinical epidemiology. 2011 Dec 1;64(12):1283-93.
Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, Alonso-Coello P, Glasziou P, Jaeschke R, Akl EA, Norris S. GRADE guidelines: 7. Rating the quality of evidence—inconsistency. Journal of clinical epidemiology. 2011 Dec 1;64(12):1294-302.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Hsu H, Siwiec RM. Knee Osteoarthritis. 2022 Sep 4. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan–. PMID: 29939661.
Huang Y, Liu X, Xu X, Liu J. Intra-articular injections of platelet-rich plasma, hyaluronic acid or corticosteroids for knee osteoarthritis. Der Orthopäde. 2019 Mar;48(3):239-47.
Jacquet C, Pioger C, Khakha R, Steltzlen C, Kley K, Pujol N, Ollivier M. Evaluation of the “Minimal Clinically Important Difference”(MCID) of the KOOS, KSS and SF-12 scores after open-wedge high tibial osteotomy. Knee Surgery, Sports Traumatology, Arthroscopy. 2021 Mar;29(3):820-6.
Johal H, Khan M, Yung SH, Dhillon MS, Fu FH, Bedi A, Bhandari M. Impact of platelet-rich plasma use on pain in orthopaedic surgery: a systematic review and meta-analysis. Sports Health. 2019 Jul;11(4):355-66.
Joshi Jubert N, Rodríguez L, Reverté-Vinaixa MM, Navarro A. Platelet-rich plasma injections for advanced knee osteoarthritis: a prospective, randomized, double-blinded clinical trial. Orthopaedic journal of sports medicine. 2017 Feb 13;5(2):2325967116689386.
Khan AF, Gillani SF, Khan AF. Role of intra-articular corticosteroid with xylocaine vs plate rich plasma for the treatment of early grade II knee osteoarthritis at Akhtar Saeed Teaching Hospital Lahore: a randomized controlled trail. Pakistan J Med Heal Sci. 2018;12:1432-5.
McLarnon M, Heron N. Intra-articular platelet-rich plasma injections versus intra-articular corticosteroid injections for symptomatic management of knee osteoarthritis: systematic review and meta-analysis. BMC musculoskeletal disorders. 2021 Dec;22(1):1-3.
Nabi BN, Sedighinejad A, Mardani-Kivi M, Haghighi M, Roushan ZA, Biazar G. Comparing the effectiveness of intra-articular platelet-rich plasma and corticosteroid injection under ultrasound guidance on pain control of knee osteoarthritis. Iranian Red Crescent Medical Journal. 2018 Mar 1;20(3).
Nie LY, Zhao K, Ruan J, Xue J. Effectiveness of platelet-rich plasma in the treatment of knee osteoarthritis: A meta-analysis of randomized controlled clinical trials. Orthopaedic Journal of Sports Medicine. 2021 Mar 2;9(3):2325967120973284.
Phul SH, Mobushir M, Jilani RU, Khan IS, Malik RH, Jan G. Comparison of intra-articular steroids injection versus platelets rich plasma injection in patients with osteoarthritic knee joints. PAKISTAN JOURNAL OF MEDICAL & HEALTH SCIENCES. 2018 Jul 1;12(3):931-4.
Velentgas P, Dreyer NA, Wu AW. Outcome Definition and Measurement. In: Velentgas P, Dreyer NA, Nourjah P, et al., editors. Developing a Protocol for Observational Comparative Effectiveness Research: A User's Guide. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013 Jan. Chapter 6. Available from: https://www.ncbi.nlm.nih.gov/books/NBK126186/
Zhang W, Doherty M, Peat G, Bierma-Zeinstra MA, Arden NK, Bresnihan B, Herrero-Beaumont G, Kirschner S, Leeb BF, Lohmander LS, Mazières B. EULAR evidence-based recommendations for the diagnosis of knee osteoarthritis. Annals of the rheumatic diseases. 2010 Mar 1;69(3):483-9.




 LOGIN
LOGIN

Join the Conversation
Please Login or Join to leave comments.
Orthopaedic Surgeon - Canada
There is no discussion on costs for use of PRP vs steroid. While cost information may not be available from the studies examined, cost information must be available in NA. This is, in my view, an important issue to be discussed especially since bias cannot be readily excluded.