
Injection of Botulinum toxin A provides pain relief for lateral epicondylitis

Injection of Botulinum toxin A provides pain relief for lateral epicondylitis
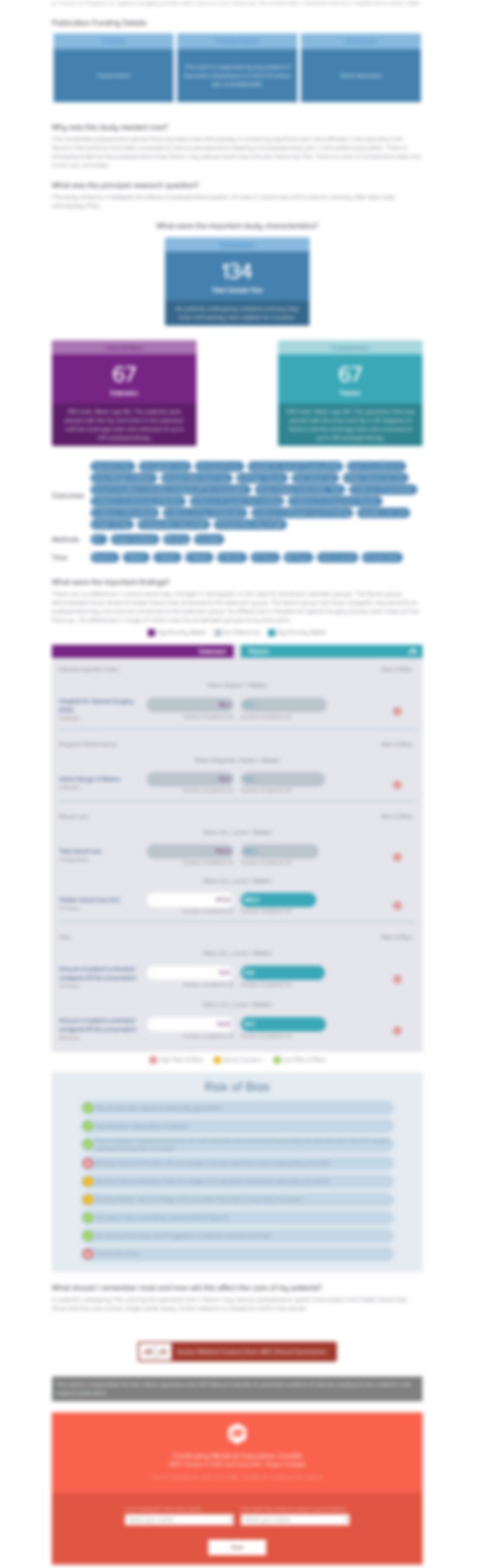
Injection of Botulinum Toxin for Treatment of Chronic Lateral Epicondylitis: Systematic Review and Meta-Analysis
Semin Arthritis Rheum. 2011 Jun;40(6):532-8. doi: 10.1016/j.semarthrit.2010.07.002. Epub 2010 Sep 6.Synopsis
This systematic review/meta-analyis utilized 10 studies (4 RCTs and 6 studies of varying quality) assessing the short term efficacy of botulinum toxin A injections into the forearm extensor muscles of patients with lateral epicondylitis. Meta-analysis utilizing the 4 included RCTs indicated that VAS pain at 4 weeks and 3 months post-intervention favoured botulinum toxin over saline solution. The r...
To view the full content, login to your account,
or start your 30-day FREE Trial today.
FREE TRIAL
LOGIN
Forgot Password?
Explore some of our unlocked ACE Reports below!
Learn about our AI Driven
High Impact Search Feature
Our AI driven High Impact metric calculates the impact an article will have by considering both the publishing journal and the content of the article itself. Built using the latest advances in natural language processing, OE High Impact predicts an article’s future number of citations better than impact factor alone.
Continue



 LOGIN
LOGIN

Join the Conversation
Please Login or Join to leave comments.