
Autologous BM-MSC Injections for Discogenic Low Back Pain

Autologous BM-MSC Injections for Discogenic Low Back Pain
Intradiscal Mesenchymal Stromal Cell Therapy for the Treatment of Low Back Pain Due to Moderate-to-Advanced Multilevel Disc Degeneration: A Preliminary Report of a Double-Blind, Phase IIB Randomized Clinical Trial (DREAM Study).
JOR Spine . 2025 Jun 2;8(2):e70086.Did you know you're eligible to earn 0.5 CME credits for reading this report? Click Here
Synopsis
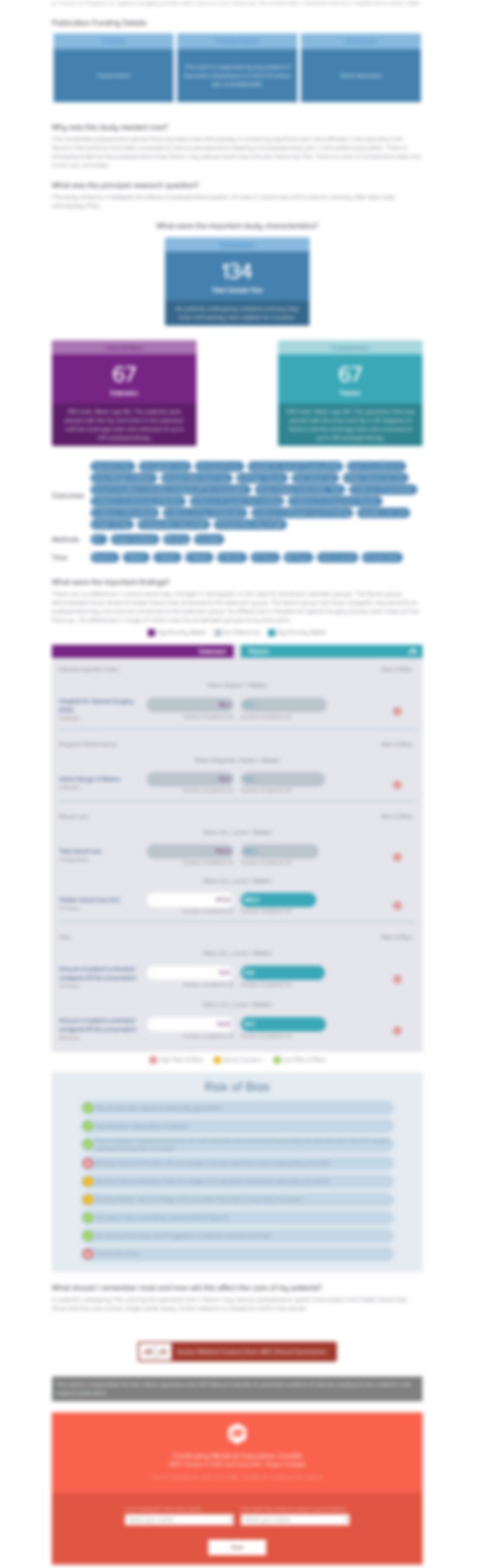
Fifty-two patients with chronic low back pain due to moderate-to-advanced intervertebral disc degeneration were randomized to receive intradiscal autologous BM-MSCs (planned n=26) or sham (planned n=26). The primary outcomes were pain (VAS) and disability (ODI). Secondary outcomes included quality of life (SF-36), work ability (WAI), and MRI measures (modified Pfirrmann grade, disc height index [D...
To view the full content, login to your account,
or start your 30-day FREE Trial today.
FREE TRIAL
LOGIN
Forgot Password?
Explore some of our unlocked ACE Reports below!
Learn about our AI Driven
High Impact Search Feature
Our AI driven High Impact metric calculates the impact an article will have by considering both the publishing journal and the content of the article itself. Built using the latest advances in natural language processing, OE High Impact predicts an article’s future number of citations better than impact factor alone.
Continue



 LOGIN
LOGIN

Join the Conversation
Please Login or Join to leave comments.