Introduction
Osteoarthritis is a leading cause of pain and disability worldwide, an estimated prevalence of 529 million people in 2020 [1]. With aging populations across the globe, this number is expected to rise over the next two decades: cases of knee osteoarthritis (KOA) are projected to increase 74.9% by 2050 [1]. Many different management strategies exist for KOA, such as exercise, physical therapy or pain medication. But, as a progressive disease with no cure, the end result is often patients requiring costly and invasive surgical treatment, such as total joint replacement [2]. This leaves health care systems around the world with an enormous economic burden that is only expected to grow [1]. Understandably, there is considerable interest in nonoperative treatment, especially those with the potential to slow or reverse the progression of the disease [2]. Enter, intraarticular injections like platelet-rich plasma (PRP).
Late 20th century pre-clinical research on PRP was encouraging [3]. Its mechanisms for promoting tissue regeneration made it a promising treatment for a variety of conditions, including KOA, where the high concentration of growth factors and cytokines could potentially counteract important aspects of KOA pathophysiology [4]. Over the last decade, clinical research on PRP has skyrocketed. However, research has largely failed to demonstrate the tissue-regenerating benefits witnessed in pre-clinical studies, and the body of evidence as a whole has suffered from significant inconsistency, poor reporting, and low quality [3].
Despite this, the use of PRP within orthopaedics has expanded at a speed rarely seen in clinical practice – a speed that has seemingly outpaced the scientific data supporting it [4]. In this OE Original, we shed light on this growing body of evidence and explore current readership, academic market, and ongoing clinical trials for PRP for KOA. Most importantly, we provide an in-depth look at what the current body of evidence says on the clinical effectiveness of PRP for KOA - courtesy of the OE Surgical Analytics platform.
“The use of intra-articular injections for treatment of knee osteoarthritis is likely to remain a topic of contentious debate and frequent publication. Further high-powered, high-quality, and nonbiased research is required to adequately address the efficacy and safety of these therapies in knee OA. Researchers, reviewers, and readers alike should keep a watchful eye for conflict of interest to ensure that patient benefit is prioritized over financial gain.” [2]
PRP for Knee Osteoarthritis
A Rise in Popularity
PRP injection use has quadrupled in the last 10 years, and is posed to increase a further 66% by 2030 [5]. Concurrently, research on its clinical effectiveness is becoming increasingly popular with orthopaedic professionals. According to readership trends identified through OE’s OrthoVision tool, two of the top 3 most read articles in the last 4 years were PRP-related (Figure 1).
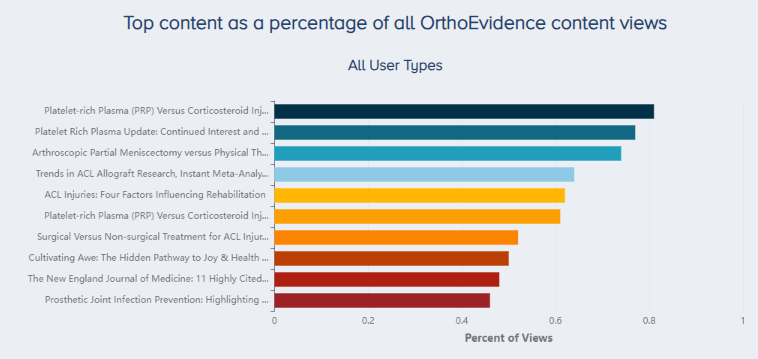 Figure 1. Most read OrthoEvidence content over the last four years. |
The top most read article over the last four years is the OE Original “Platelet-rich Plasma (PRP) Versus Corticosteroid Injection for Knee Osteoarthritis: A Systematic Review of Randomized Controlled Trials” (Link). The second most read article was the OE Insight “Platelet Rich Plasma Update: Continued Interest and a Call for Change Including Insights From OE Mind and OE Vision” (Link). The sixth most-read article was also related to PRP, and is another OE Original publication titled “Platelet-rich Plasma (PRP) Versus Corticosteroid Injection for Painful Shoulder Conditions: Powered by OE M.I.N.D.” (Link).
Market Analysis
Global interest in PRP is at an all-time high, and this is reflected in the size and growth of the market. The global PRP market is projected to grow to USD 1.94 billion dollars by 2030, representing a compound annual growth rate of 15.1% from 2023-2030 [6]. This makes it a promising space for orthopaedic companies looking to expand their offerings and take advantage of a growing market.
The academic literature is a place where companies can not only demonstrate, but advertise the effectiveness of their products. By using OE’s Market Analysis tool, we can determine who is dominating the academic literature, and subsequently controlling the narrative. Within this tool, we present power rankings, a calculation of the research impact of companies, to determine which companies have the largest academic footprint in a given treatment or condition.
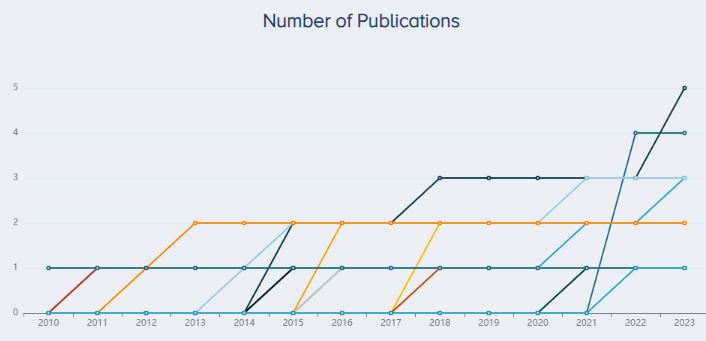
We identified 70 studies investigating PRP for KOA involving over 7,000 patients. As of December 7th, 2023, the company with the largest footprint in PRP research for KOA was Regen Lab (makers of Regenkit-BCT), followed by TCM Biotech (makers of PLTenus Plus and Verte PLT Plus), Arya Mabna Tashkis (makers of Rooyagen), Sigma, and Arthrex (makers of Arthrex ACP Max and Arthrex ACP Double Syringe System) (Figure 2).
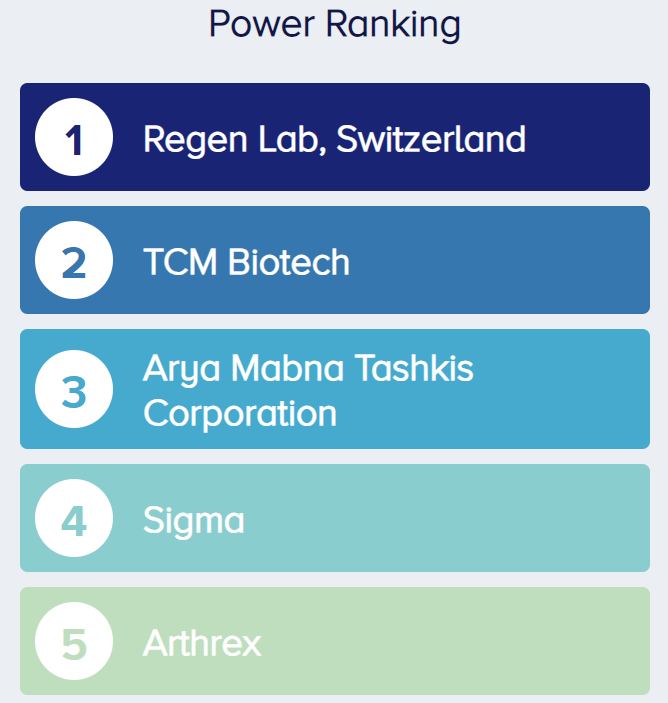 Figure 2. The OE Power Ranking shows the five companies with the largest impact on PRP for KOA research. |
The companies with the most publications are Regen Lab (5 publications), TCM Biotech (4), Arthrex (3), and Arya Mabna Tashkis (3) followed by Sigma, Becton Dickinson, BTI Biotechnology Institute, all with two publications (Figure 3).
|
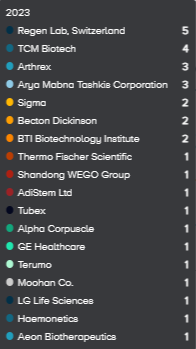 Figure 3. Companies with the highest number of publications for PRP for KOA research. |
The companies with the largest sample size across all trials are Sigma (200 patients), Arya Mabna Tashkis (177), TCM Biotech (145), Regen Lab (139) and BTI Biotechnology (137) (Figure 4).
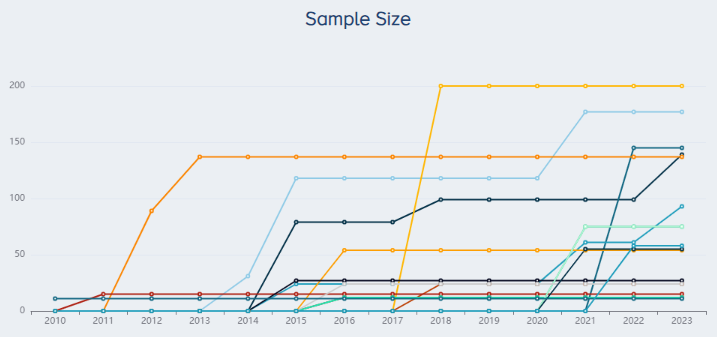 |
 Figure 4. Companies with the largest research sample size for PRP for KOA research. |
The countries where the most PRP research has been published are China (10 studies), followed by Iran (9) and Turkey (7) (Figure 5).
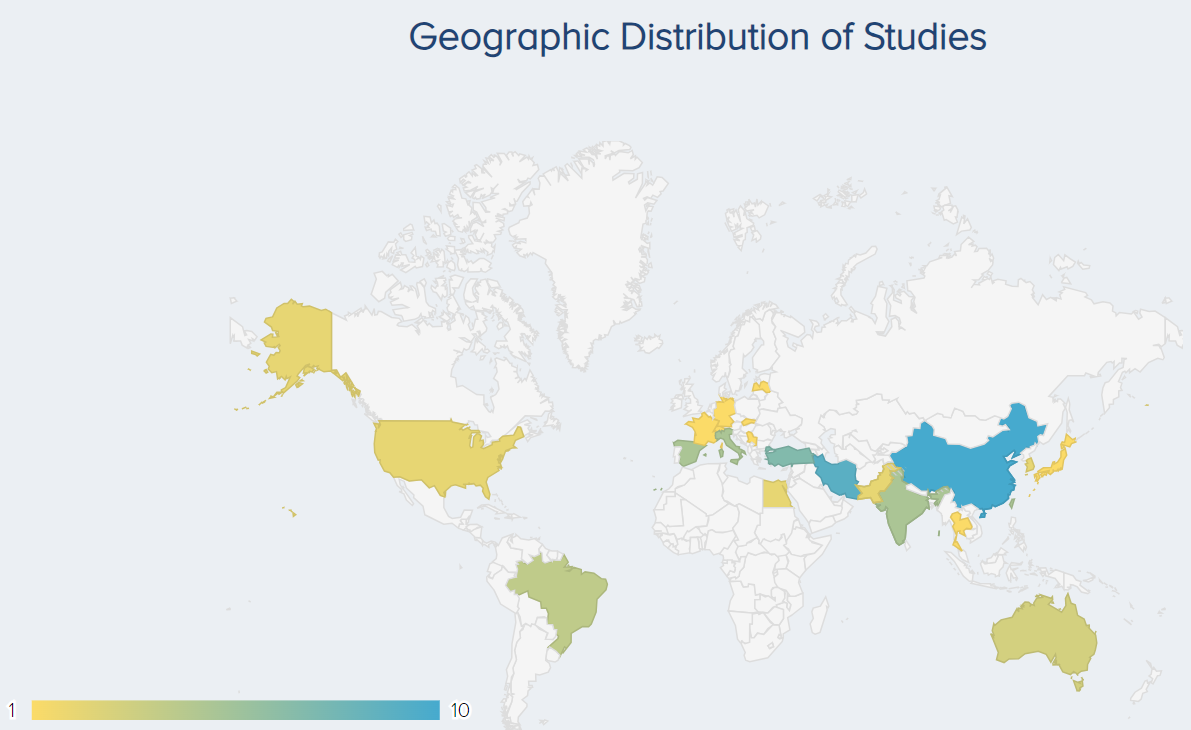 Figure 5. Geographic distribution of studies for PRP for KOA research. |
Ongoing Trials
The Surgical Analytics platform gives you a complete picture of the research: past, present, and future. While the Market Analysis tool breaks down the current academic marketplace and the key companies in the space, the Ongoing Trials tool, powered by ClinicalTrials.gov, looks into the future to tell you what the future of PRP research looks like.
As of December 7th, 2023, there were 16 ongoing interventional clinical trials involving 588 patients (Figure 6). The country where the most studies are being conducted is Taiwan (3 studies), followed by the United States (2 studies) (Figure 7).
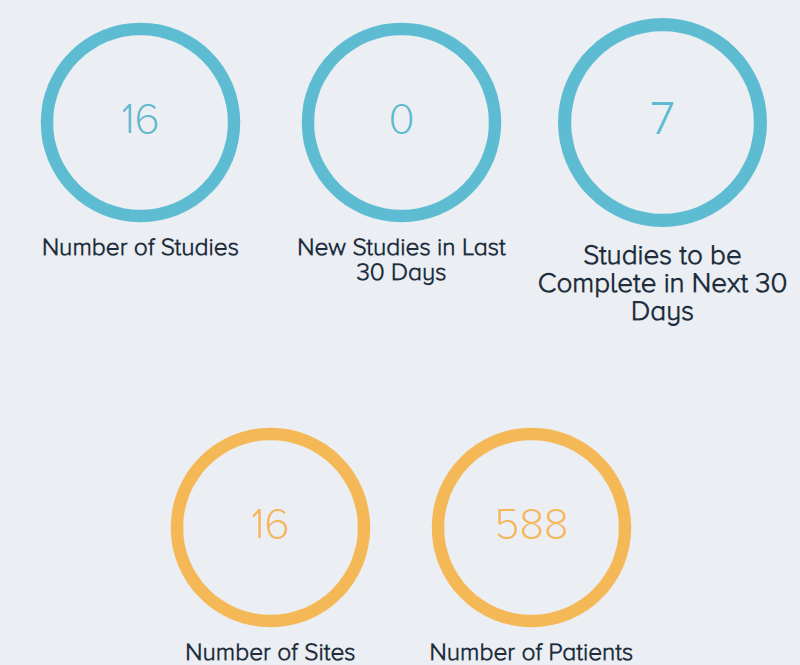 Figure 6. An overview of the ongoing PRP for KOA trials. |
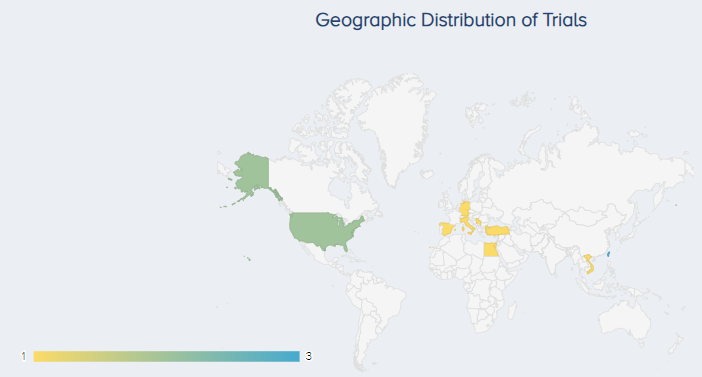 Figure 7. The geographic distribution of ongoing PRP for KOA trials. |
Clinical Effectiveness
Does the evidence support the use of PRP for KOA? Yes and no. While there are studies that support the use of PRP for KOA, the true clinical efficacy of PRP is dependent on many factors, including the method of preparation (use of an open vs closed preparation method, use of the single- vs double-spinning technique, speed and duration of centrifugation, whether an activator was used), administration (number of injection, frequency of PRP injections, injection volume) and final composition (platelet concentration, high or low leukocyte count) of PRP used [7-10]. These factors may lead to the creation of PRP preparations with differing biological activity, resulting in different physiological responses in patients [7-10]. This could explain the inconsistent results of clinical trials investigating PRP for KOA. Currently, clinical guidelines do not recommend its use [11], or do so on a very limited basis [12,13].
Using OE’s Meta-Analyzer and Forecaster tools, we assess what the current body of evidence suggests for the clinical efficacy and safety of PRP versus saline, HA, and corticosteroids for KOA. All analyses were performed on December 7, 2023.
1. PRP vs Saline
How effective is PRP compared to placebo? A meta-analysis by Dai et al. (2016) found that PRP was superior to saline in reducing pain and functional improvement at 6 and 12 months post-injection [10].
Using the OE Meta-Analyzer tool, we identified 12 studies involving 1,323 patients. The results were similar to those demonstrated by Dai et al. (2016), with PRP resulting in a significant improvement in function and pain (Figures 8 & 9) and no significant difference in incidence of adverse events (Figure 10).
Function: For the analysis of function, 8 RCTs including a total of 736 patients comparing PRP to normal saline for the treatment of KOA were identified. The overall effect demonstrates that PRP results in a significant improvement in function with patients experiencing, on average, an 11.51 (5.69 to 17.32 95% CI) point improvement (Figure 8).
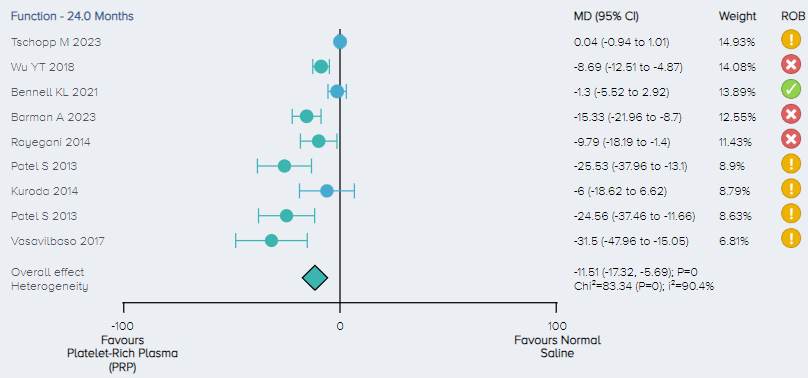 Figure 8. Forest plot comparing functional outcomes on a normalized 0-100 scale for PRP versus control. Notes: ROB = risk of bias; red circle with a cross mark = high risk of bias; yellow circle with an exclamation mark = have some concerns; green circle with a checkmark = low risk of bias. |
Pain: For the analysis of pain, 11 RCTs including a total of 1,141 patients comparing PRP to normal saline for the treatment of KOA were identified. Pain scores were normalized on a 1-100 scale. The overall effect demonstrates that PRP results in a significant improvement in pain with patients experiencing, on average, a 5.73 (3.36 to 8.11 95% CI) point improvement (Figure 9).
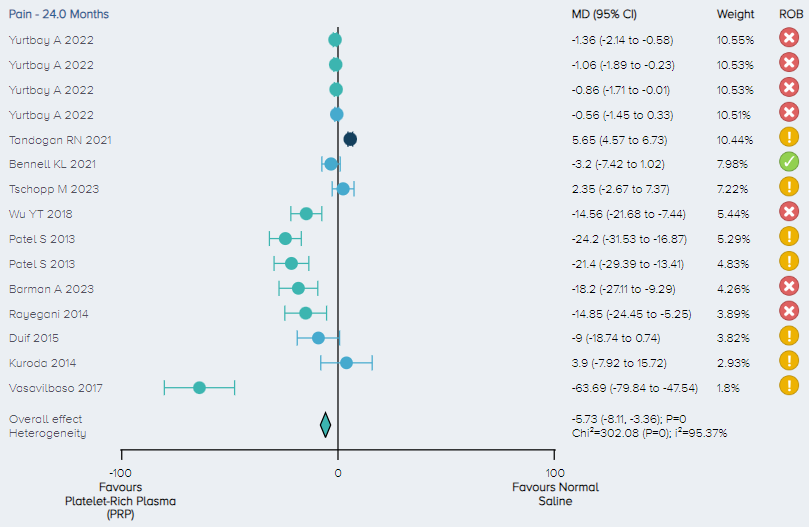 Figure 9. Forest plot comparing pain outcomes on a normalized 0-100 scale for PRP versus control. Notes: ROB = risk of bias; red circle with a cross mark = high risk of bias; yellow circle with an exclamation mark = have some concerns; green circle with a checkmark = low risk of bias. |
Adverse events: For the analysis of the incidence of adverse events, 3 studies including a total of 148 patients comparing PRP to control for the treatment of KOA were identified. There was no significant difference in the incidence of adverse events between PRP and control (Figure 10).
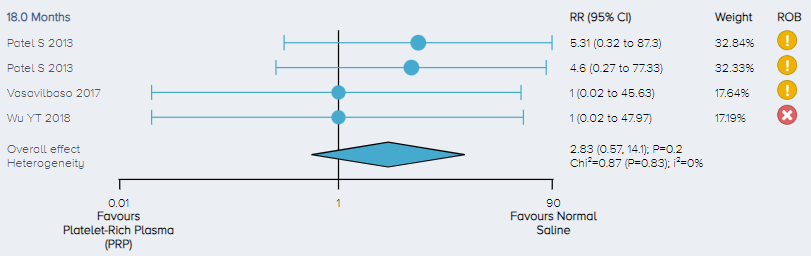 Figure 10. Forest plot comparing the incidence of adverse events for PRP versus control. Notes: ROB = risk of bias; red circle with a cross mark = high risk of bias; yellow circle with an exclamation mark = have some concerns; green circle with a checkmark = low risk of bias. |
2. PRP versus HA
How does PRP compare to other popular injectable treatments for KOA? To start, we compare PRP to HA - another popular injectable for KOA with mixed reviews. Like PRP, it has also been recommended against in the American College of Rheumatology 2019 Osteoarthritis Guideline [11]. A systematic review and meta-analysis by Atef et al. (2021) comparing PRP to HA found that the intra-articular injection of PRP was more effective in terms of pain relief and functional improvement at short-term follow-up, while no significant difference was found in the risk of adverse events [14].
Using the OE Meta-Analyzer Tool, we identified 29 studies involving 3,493 patients that compared PRP to HA for KOA. Like Atef et al. (2021), we found PRP resulted in a significant improvement in function and pain compared to HA (Figure 11 & 12) and found no significant difference in incidence of adverse events (Figure 13).
Function: For the analysis of function, 20 RCTs including a total of 2,354 patients comparing PRP and HA for the treatment of KOA were identified. Function scores were normalized on a 1-100 scale. The overall effect demonstrates that PRP results in a significant improvement in function with patients experiencing, on average, a 5.93 (3.07 to 8.79 95% CI) point improvement. (Figure 11).
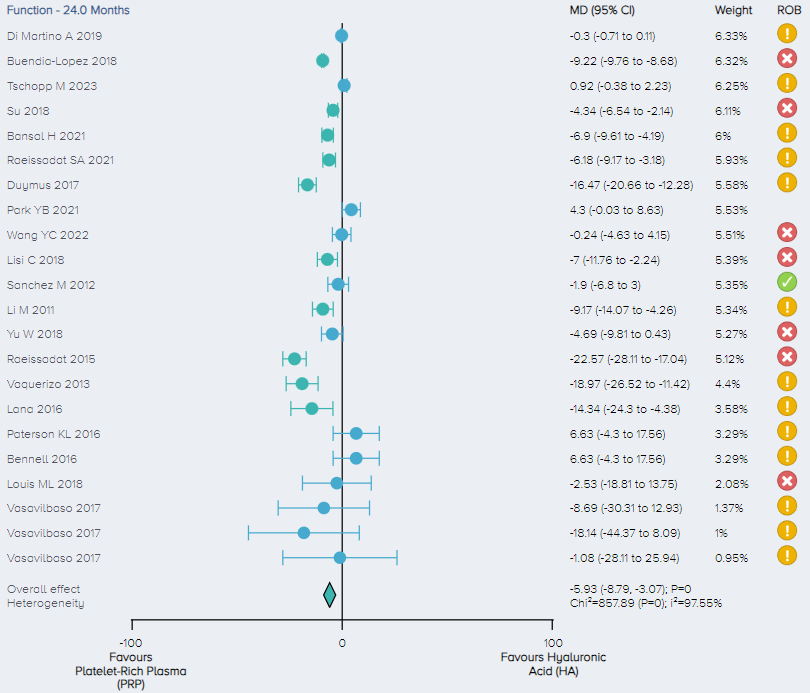 Figure 11. Forest plot comparing functional outcomes between PRP and HA on a normalized 0-100 scale. Notes: ROB = risk of bias; red circle with a cross mark = high risk of bias; yellow circle with an exclamation mark = have some concerns; green circle with a checkmark = low risk of bias. |
Pain: For the analysis of pain, 25 RCTs including a total of 2,969 patients comparing PRP and HA for the treatment of KOA were identified. The overall effect demonstrates that PRP results in a significant improvement in pain with patients experiencing, on average, a 3.11 (1.94 to 4.28 95% CI) point improvement. (Figure 12).
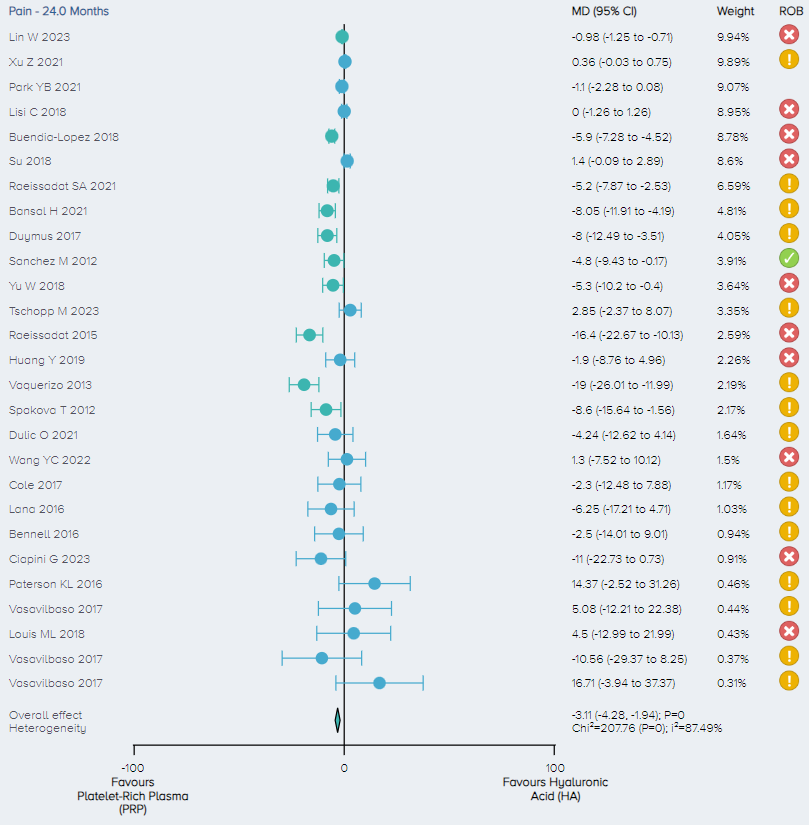 Figure 12. Forest plot comparing pain outcomes between PRP and HA on a normalized 0-100 scale. Notes: ROB = risk of bias; red circle with a cross mark = high risk of bias; yellow circle with an exclamation mark = have some concerns; green circle with a checkmark = low risk of bias. |
Adverse events: For the analysis of the incidence of adverse events, 8 studies including a total of 573 patients comparing PRP to HA for the treatment of KOA were identified. There was no significant difference in the incidence of adverse events between PRP and control (Figure 13).
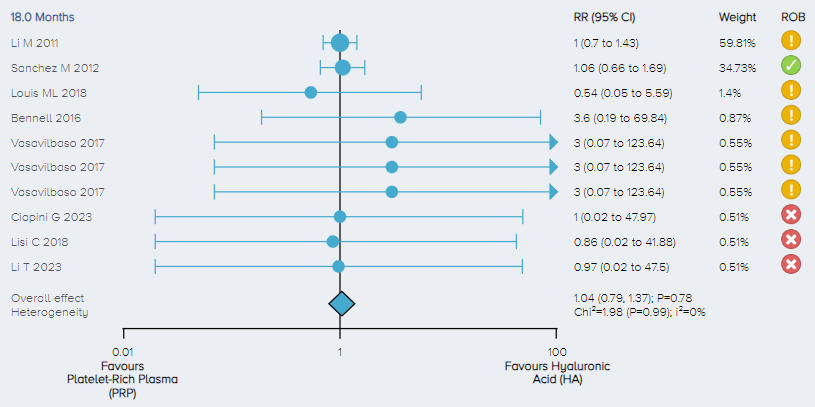 Figure 13. Forest plot comparing the incidence of adverse events for PRP versus HA. Notes: ROB = risk of bias; red circle with a cross mark = high risk of bias; yellow circle with an exclamation mark = have some concerns; green circle with a checkmark = low risk of bias. |
3. PRP versus Corticosteroid:
Next we look at how PRP compares to another popular injectable treatment for KOA: corticosteroids. According to the NICE guidelines for osteoarthritis, corticosteroids have demonstrated inconsistent benefits in patients with KOA [12]. The guideline recommends the use of corticosteroids in more of a supporting role, used alongside therapeutic exercise when other pharmacological treatments have failed [12]. A systematic review and meta-analysis by McLarnen et al. (2021) found that the intraarticular injection of PRP improved pain, and resulted in less joint stiffness and better return to exercise and sporting activities at 12 months compared to the intraarticular injection of corticosteroids. [15]
Using the OE Meta-Analyzer Tool, we identified 9 studies involving 731 patients that compared PRP to corticosteroid for KOA. Similar to McLarnen et al. (2021), we found PRP resulted in a significant improvement in function and pain (Figures 14 & 15). No significant difference in incidence of adverse events was found between PRP and corticosteroid (Figure 16).
Function: For the analysis of function, 7 RCTs including a total of 531 patients comparing PRP and corticosteroid for the treatment of KOA were identified. Function scores were normalized on a 1-100 scale. The overall effect demonstrates that PRP results in a significant improvement in function with patients experiencing, on average, a 10.16 (2.16 to 18.15 95% CI) point improvement. (Figure 14)
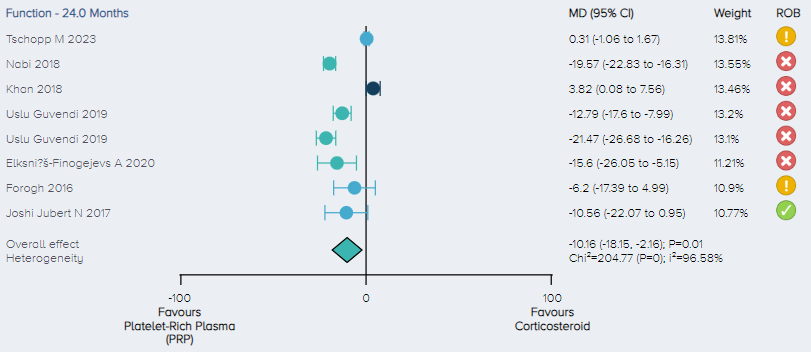 Figure 14. Forest plot comparing functional outcomes between PRP and corticosteroid on a normalized 0-100 scale. Notes: ROB = risk of bias; red circle with a cross mark = high risk of bias; yellow circle with an exclamation mark = have some concerns; green circle with a checkmark = low risk of bias. |
Pain: For the analysis of pain, 3 RCTs including a total of 257 patients comparing PRP and corticosteroid for the treatment of KOA were identified. The overall effect demonstrates that PRP results in a significant improvement in pain with patients experiencing, on average, a 12.32 (3.31 to 21.32 95% CI) point improvement. (Figure 15).
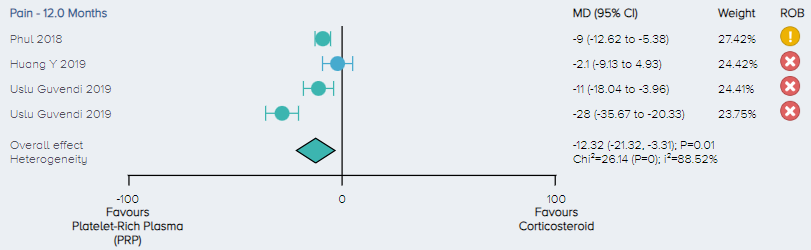 Figure 15. Forest plot comparing pain outcomes between PRP and corticosteroid on a normalized 0-100 scale. Notes: ROB = risk of bias; red circle with a cross mark = high risk of bias; yellow circle with an exclamation mark = have some concerns; green circle with a check mark = low risk of bias. |
Adverse events: For the analysis of the incidence of adverse events, 2 studies including a total of 105 patients comparing PRP to corticosteroid for the treatment of KOA were identified. There was no significant difference in the incidence of adverse events between PRP and corticosteroid (Figure 16).
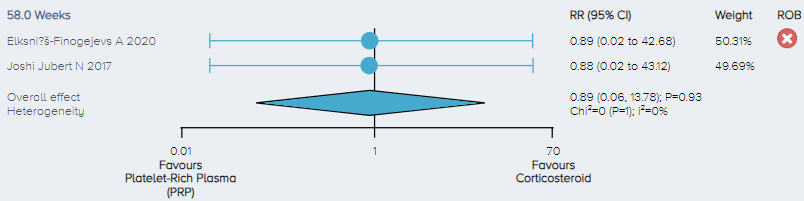 Figure 16. Forest plot comparing the incidence of adverse events for PRP versus corticosteroid. Notes: ROB = risk of bias; red circle with a cross mark = high risk of bias; yellow circle with an exclamation mark = have some concerns; green circle with a checkmark = low risk of bias. |
Final Thoughts
Our results suggest that PRP significantly improved function and pain compared to saline, corticosteroids and HA. No difference was found in terms of incidence of adverse events for any of these comparisons. These results are in line with previously published meta-analyses.
There appears to be some promise when it comes to PRP as a treatment for KOA. However, as concluded by many RCTs, meta-analyses, orthopaedic blogs, and clinical guidelines worldwide, this evidence is highly heterogeneous and provides insufficient evidence to support its widespread use. More high-quality research with longer-term follow-up is greatly needed. Additionally, improved reporting is a must, particularly with regard to the composition of the PRP used (including leukocyte concentration) and the methods used to prepare it. More research also needs to be aimed at determining which patient populations PRP may be most effective for, and the compositions that are best suited to serve them. With these things in mind, there is hope that future research will be able to provide answers to critical questions that will help to reveal the true potential of PRP within orthopaedics.
References
- GBD 2021 Osteoarthritis Collaborators. Global, regional, and national burden of osteoarthritis, 1990-2020 and projections to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023;5(9):e508-e522. Published 2023 Aug 21. https://doi.org/10.1016/S2665-9913(23)00163-7
- Paterson K (2022, January 13). Cutting through the hype on platelet-rich plasma. Pursuit. Retrieved from: https://pursuit.unimelb.edu.au/articles/cutting-through-the-hype-on-platelet -rich-plasma
- Moscicka P, Przylipiak A. History of autologous platelet-rich plasma: A short review. J Cosmet Dermatol. 2021;20(9):2712-2714. https://doi.org/10.1111/jocd.14326
- Simental-Mendía M, Ortega-Mata D, Acosta-Olivo CA. Platelet-rich plasma for knee osteoarthritis: What does the evidence say? Drugs Aging. 2023;40: 585–603. https://doi.org/10.1007/s40266-023-01040-6
- Berlinberg EJ, Swindell H, Patel HH, et al. The Epidemiology of Platelet-Rich Plasma Injections From 2010 to 2020 in a Large US Commercial Insurance Claims Database: A Recent Update. J Am Acad Orthop Surg. 2023;31(3):e135-e147. https://doi.org/10.5435/JAAOS-D-22-00397
- Business Wire (2023, July 13). Global platelet rich plasma (PRP) market trends/analysis report 2023-2030. Business Wire. Retrieved from: https://www.businesswire.com/news/home/ 20230713875191/en/Global-Platelet-Rich-Plasma-PRP-Market-TrendsAnalysis-Report-2023-2030-The-1.94-Billion-Market-is-Poised-for-Strong-Growth-Driven-by-Rising-Demand-in-Cosmetic-Surgeries-and-Sports-Injuries---ResearchAndMarkets.com
- Atef ME, Zeiad MZ, Mohamed ASI. Intra-articular injections of platelet-rich plasma versus hyaluronic acid in treatment of knee osteoarthritis (Systematic Review and Meta IFAAS. PRP Preparation: Open or closed technique? International Fellowship in Advanced Aesthetic Science. Accessed November 28th, 2023. Retrieved from: https://www.ifaas.co/single-post/prp-preparation-open-or-closed-technique
- Dashore S, Chouhan K, Nanda S, Sharma A. Preparation of Platelet-Rich Plasma: National IADVL PRP Taskforce Recommendations. Indian Dermatol Online J. 2021;12(Suppl 1):S12-S23. https://doi.org/10.4103/idoj.idoj_269_21
- Mazzocca AD, McCarthy MBR, Chowaniec DM, Cote MP, Romeo AA, Bradley JP, Arciero RA, Beitzel K. Platelet-rich plasma differs according to preparation method and human variability. The Journal of Bone & Joint Surgery. 2012;94(4): 308-316.
- Dai WL, Zhou AG, Zhang H, Zhang J. Efficacy of Platelet-Rich Plasma in the Treatment of Knee Osteoarthritis: A Meta-analysis of Randomized Controlled Trials. Arthroscopy. 2017;33(3):659-670.e1. https://doi.org/10.1016/j.arthro.2016.09.024
- https://assets.contentstack.io/v3/assets/bltee37abb6b278ab2c/blt6aa092f0134cac9a/63320f4750c8e90e3bf512c2/osteoarthritis-guideline-2019.pdf
- https://www.nice.org.uk/guidance/ipg637/chapter/1-Recommendations
- https://www.aaos.org/globalassets/quality-and-practice-resources/biologics/technology-overview_prp-for-knee-oa.pdf
- Atef ME, Zeiad MZ, Mohamed ASI. Intra-articular injections of platelet-rich plasma versus hyaluronic acid in treatment of knee osteoarthritis (Systematic Review and Meta-analysis). QJM: An International Journal of Medicine. 2021; 114(1). https://doi.org/10.1093/qjmed/hcab104.014
- McLarnon M, Heron N. Intra-articular platelet-rich plasma injections versus intra-articular corticosteroid injections for symptomatic management of knee osteoarthritis: systematic review and meta-analysis. BMC Musculoskelet Disord. 2021;22(1):550. Published 2021 Jun 16. https://doi.org/10.1186/s12891-021-04308-3




 LOGIN
LOGIN

Join the Conversation
Please Login or Join to leave comments.