
Nadroparin or fondaparinux for thromboprophylaxis after ankle/foot fracture managed conservatively

Nadroparin or fondaparinux for thromboprophylaxis after ankle/foot fracture managed conservatively
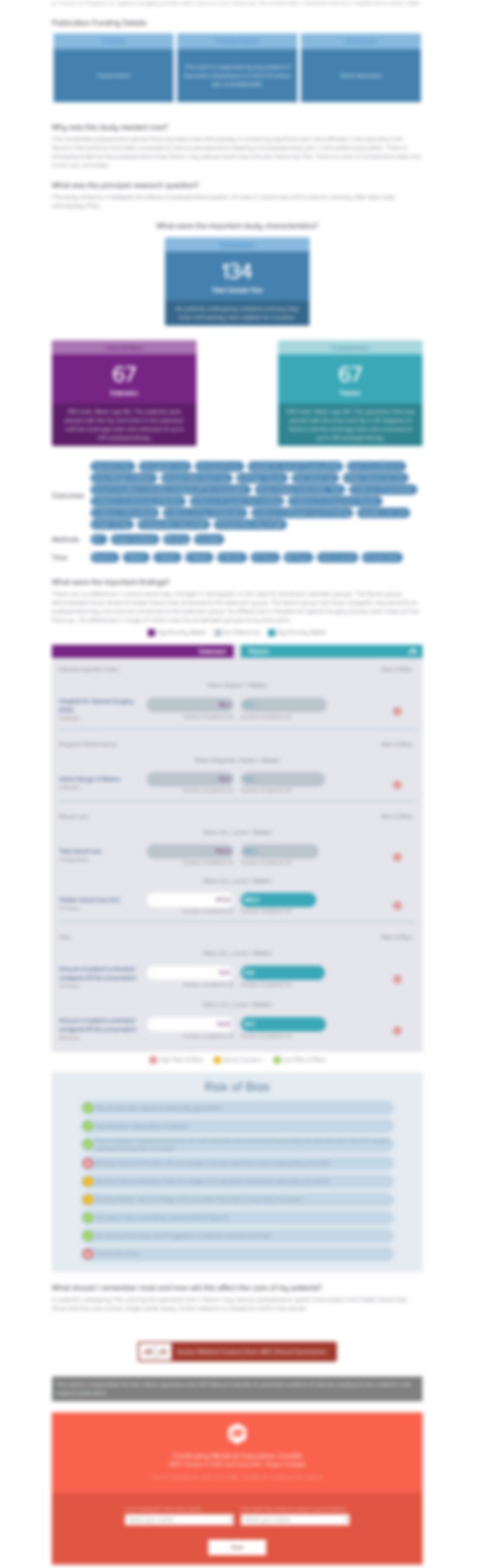
Nadroparin or fondaparinux versus no thromboprophylaxis in patients immobilised in a below-knee plaster cast (PROTECT): A randomised controlled trial
Injury. 2017 Apr;48(4):936-940Synopsis
467 patients managed with a below-knee plaster cast for a foot or ankle fracture were randomized to either subcutaneous nadroparin 2850IE daily, subcutaneous fondaparinux 2.5mg daily, or no thromboprophylaxis intervention (control). Patients were screened during follow-up for cases of deep vein thrombosis and pulmonary embolism. Results demonstrated significantly lower rates of deep vein thrombosi...
To view the full content, login to your account,
or start your 30-day FREE Trial today.
FREE TRIAL
LOGIN
Forgot Password?
Explore some of our unlocked ACE Reports below!
Learn about our AI Driven
High Impact Search Feature
Our AI driven High Impact metric calculates the impact an article will have by considering both the publishing journal and the content of the article itself. Built using the latest advances in natural language processing, OE High Impact predicts an article’s future number of citations better than impact factor alone.
Continue



 LOGIN
LOGIN

Join the Conversation
Please Login or Join to leave comments.