
OARSI 2016: IA 20mg & 40mg extended-release triamcinolone acetonide vs placebo for knee OA

OARSI 2016: IA 20mg & 40mg extended-release triamcinolone acetonide vs placebo for knee OA
Sustained and profound analgesic benefits in people with osteoarthritis of the knee using FX006, an intra-articular extended-release formulation of triamcinolone acetonide: results from a double-blind, randomized, parallel-group, dose-ranging study
Did you know you're eligible to earn 0.5 CME credits for reading this report? Click Here
CONFERENCE ACE REPORTS
This ACE Report is a summary of a conference presentation or abstract. The information provided has limited the ability to provide an accurate assessment of the risk of bias or the overall quality. Please interpret the results with caution as trials may be in progress and select results may have been presented.
Synopsis
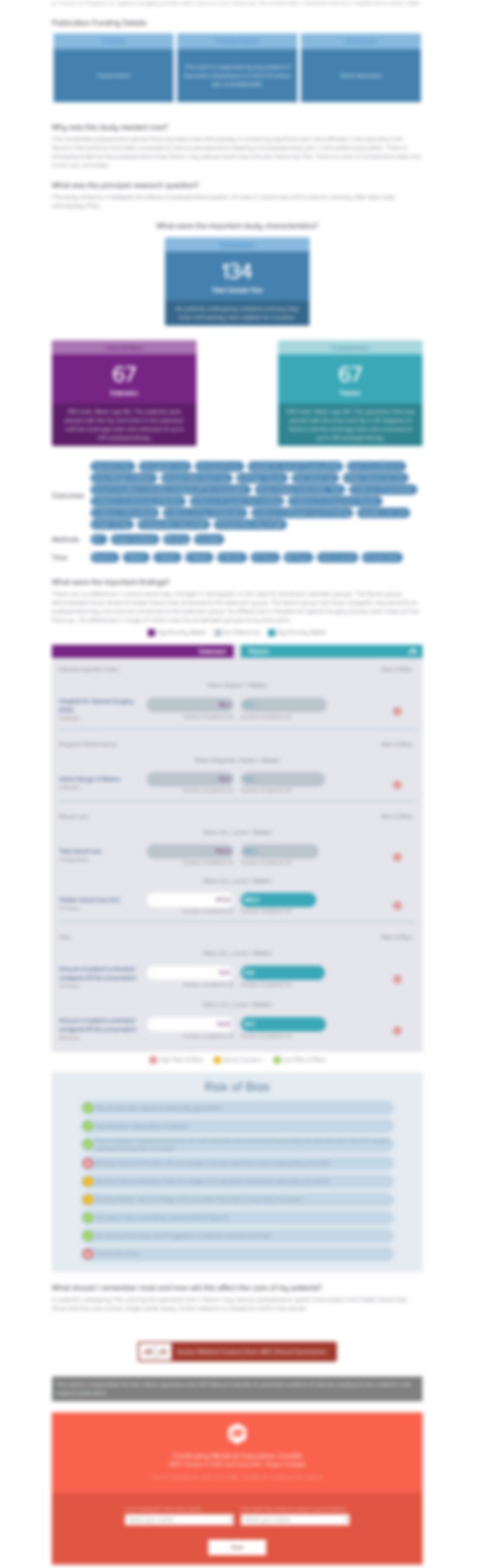
306 patients with Kellgren-Lawrence grade II-III knee osteoarthritis were randomized to one of three groups for intra-articular injection: 20mg extended-release triamcinolone acetonide, 40mg extended-release acetonide (ER TCA), or placebo. The purpose of this study was to examine and compare the efficacy of groups based on pain reduction relative to baseline over the first 24 weeks post-injection....
To view the full content, login to your account,
or start your 30-day FREE Trial today.
FREE TRIAL
LOGIN
Forgot Password?
Explore some of our unlocked ACE Reports below!
Learn about our AI Driven
High Impact Search Feature
Our AI driven High Impact metric calculates the impact an article will have by considering both the publishing journal and the content of the article itself. Built using the latest advances in natural language processing, OE High Impact predicts an article’s future number of citations better than impact factor alone.
Continue


 LOGIN
LOGIN

Join the Conversation
Please Login or Join to leave comments.