
Interspinous process device not favorable over decompression for LSS at 2 years

Interspinous process device not favorable over decompression for LSS at 2 years
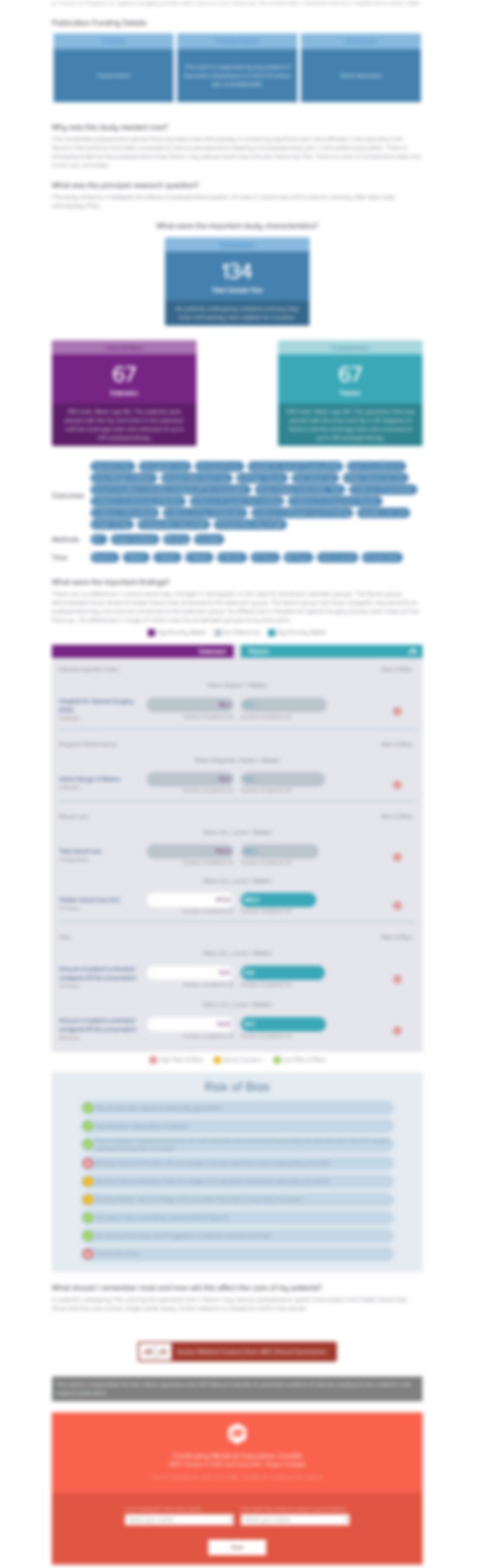
IPD without bony decompression versus conventional surgical decompression for lumbar spinal stenosis: 2-year results of a double-blind randomized controlled trial
Eur Spine J. 2015 Oct;24(10):2295-305Synopsis
159 patients with intermittent neurogenic claudication due to single- or two-level degenerative lumbar spinal stenosis were selected for inclusion. The purpose of this randomized controlled trial was to determine how treatment with an interspinous process device without bony decompression compared to bony decompression 2 years post-operatively. At 2 years, there was no significant difference betwe...
To view the full content, login to your account,
or start your 30-day FREE Trial today.
FREE TRIAL
LOGIN
Forgot Password?
Explore some of our unlocked ACE Reports below!
Learn about our AI Driven
High Impact Search Feature
Our AI driven High Impact metric calculates the impact an article will have by considering both the publishing journal and the content of the article itself. Built using the latest advances in natural language processing, OE High Impact predicts an article’s future number of citations better than impact factor alone.
Continue


 LOGIN
LOGIN

Join the Conversation
Please Login or Join to leave comments.