
Effect of tranexamic acid and indirect factor Xa inhibitor on blood loss and DVT after TKA

Effect of tranexamic acid and indirect factor Xa inhibitor on blood loss and DVT after TKA
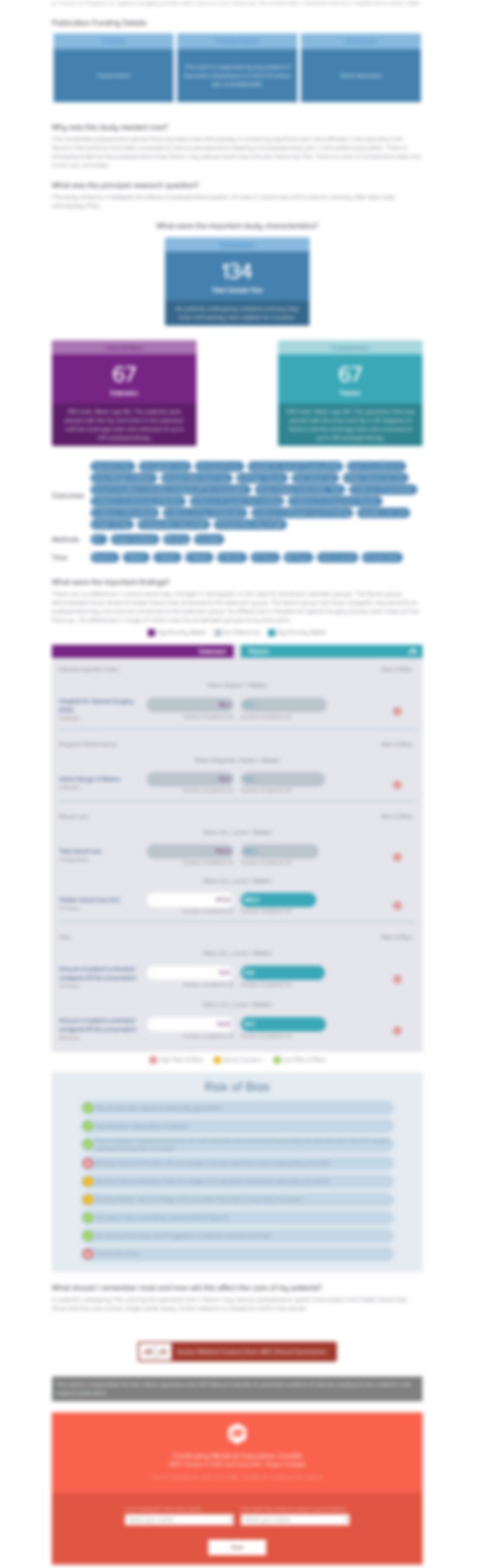
Less blood loss under concomitant administration of tranexamic acid and indirect factor Xa inhibitor following total knee arthroplasty: a prospective randomized controlled trial
Knee Surg Sports Traumatol Arthrosc. 2013 Nov;21(11):2611-7Did you know you're eligible to earn 0.5 CME credits for reading this report? Click Here
Synopsis
72 patients scheduled to receive primary unilateral cemented total knee arthroplasty (TKA) were randomly assigned into one of two groups to determine if the simultaneous application of tranexamic acid and indirect factor Xa inhibitor was a safe and effective blood loss prevention treatment when compared to indirect factor Xa inhibitor alone. Patients either received tranexamic acid in combination ...
To view the full content, login to your account,
or start your 30-day FREE Trial today.
FREE TRIAL
LOGIN
Forgot Password?
Explore some of our unlocked ACE Reports below!
Learn about our AI Driven
High Impact Search Feature
Our AI driven High Impact metric calculates the impact an article will have by considering both the publishing journal and the content of the article itself. Built using the latest advances in natural language processing, OE High Impact predicts an article’s future number of citations better than impact factor alone.
Continue



 LOGIN
LOGIN

Join the Conversation
Please Login or Join to leave comments.