
Risedronate reduces fracture risk in children with osteogenesis imperfecta

Risedronate reduces fracture risk in children with osteogenesis imperfecta
Risedronate in children with osteogenesis imperfecta: a randomised, double-blind, placebo-controlled trial
Lancet. 2013 Aug 5. pii: S0140-6736(13)61091-0.Did you know you're eligible to earn 0.5 CME credits for reading this report? Click Here
Synopsis
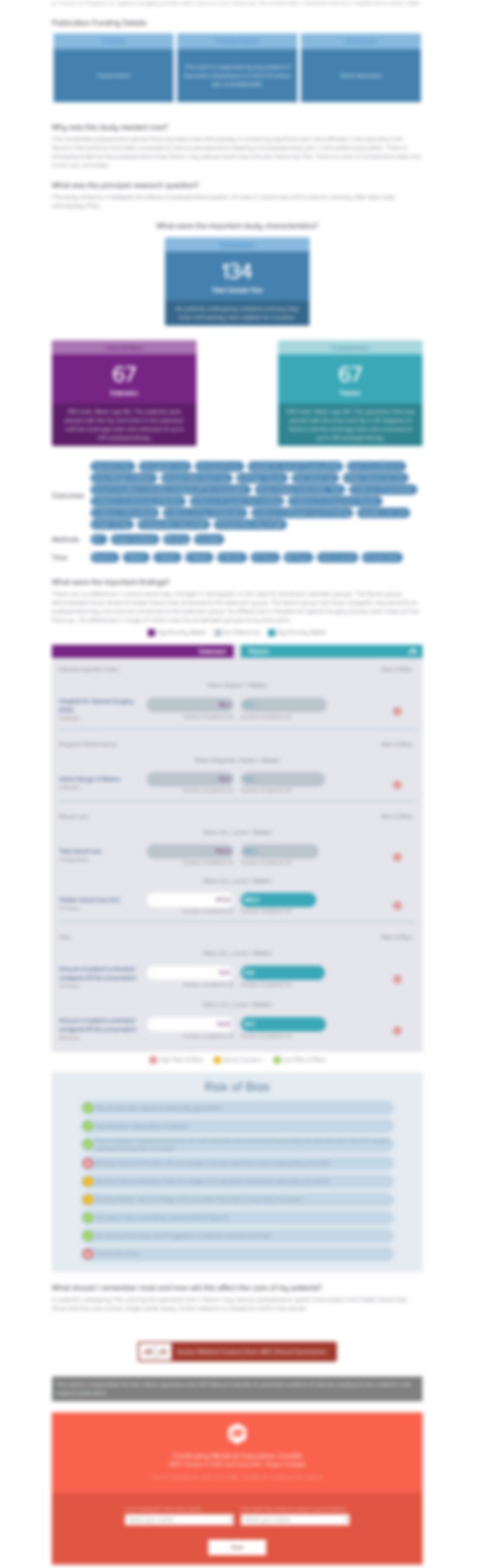
147 children with osteogenesis imperfecta were randomized to receive risedronate (an oral bisphosphonate) or a placebo in order to evaluate the safety and efficacy of risedronate in this population. The study demonstrated that risedronate was superior in the mean improvement in lumbar spinal BMD- assessed using dual-energy x-ray absorptiometry scanning, and led to a reduced risk of clinical fractu...
To view the full content, login to your account,
or start your 30-day FREE Trial today.
FREE TRIAL
LOGIN
Forgot Password?
Explore some of our unlocked ACE Reports below!
Learn about our AI Driven
High Impact Search Feature
Our AI driven High Impact metric calculates the impact an article will have by considering both the publishing journal and the content of the article itself. Built using the latest advances in natural language processing, OE High Impact predicts an article’s future number of citations better than impact factor alone.
Continue



 LOGIN
LOGIN

Join the Conversation
Please Login or Join to leave comments.