
MTP-PE added to chemotherapy did not improve outcomes in metastatic osteosarcoma patients

MTP-PE added to chemotherapy did not improve outcomes in metastatic osteosarcoma patients
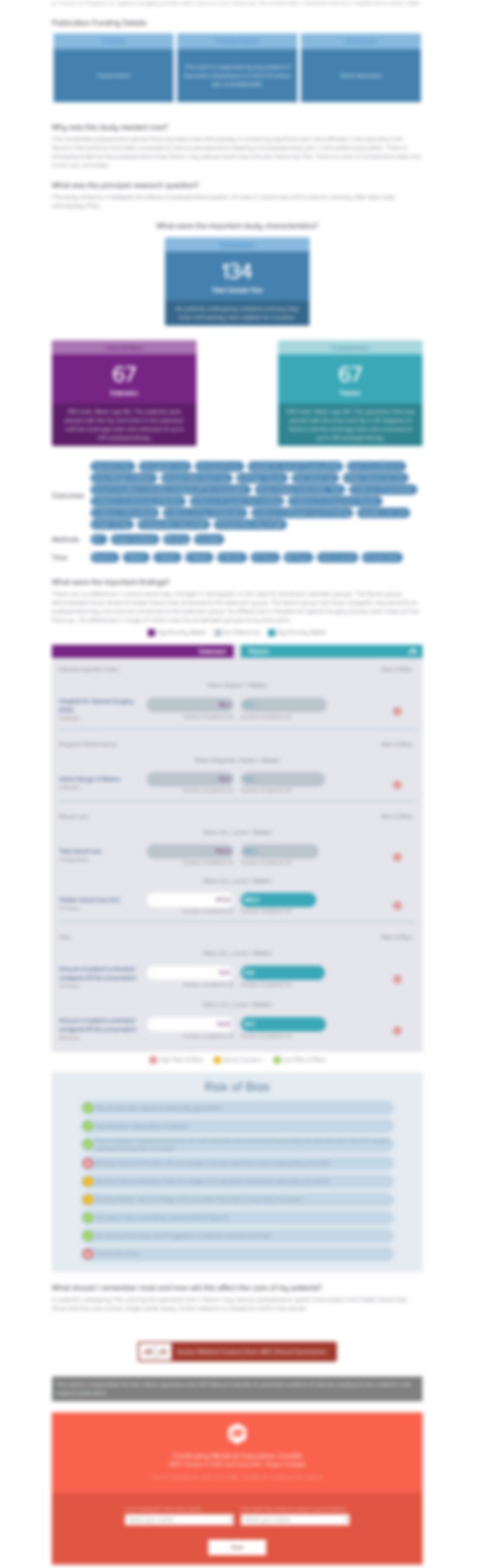
Addition of muramyl tripeptide to chemotherapy for patients with newly diagnosed metastatic osteosarcoma: a report from the Children's Oncology Group
Cancer. 2009 Nov 15;115(22):5339-48. doi: 10.1002/cncr.24566Synopsis
91 patients with metastatic osteosarcoma were randomized to receive a 3-drug treatment of chemotherapy (cisplatin, doxorubicin, and high-dose methotrexate) with or without ifosfamide. Within each group patients were further randomized to either receive liposomal muramyl tripeptide phosphatidylethanolamine (MTP-PE) or not to determine whether the addition of liposomal MTP-PE to chemotherapy was eff...
To view the full content, login to your account,
or start your 30-day FREE Trial today.
FREE TRIAL
LOGIN
Forgot Password?
Explore some of our unlocked ACE Reports below!
Learn about our AI Driven
High Impact Search Feature
Our AI driven High Impact metric calculates the impact an article will have by considering both the publishing journal and the content of the article itself. Built using the latest advances in natural language processing, OE High Impact predicts an article’s future number of citations better than impact factor alone.
Continue



 LOGIN
LOGIN

Join the Conversation
Please Login or Join to leave comments.