
Efficacy & Safety of Topical Tranexamic Acid in Elderly Hip Fractures Undergoing Surgical Treatment

Efficacy & Safety of Topical Tranexamic Acid in Elderly Hip Fractures Undergoing Surgical Treatment
Efficacy and Safety of Topical Tranexamic Acid in Elderly Hip Fractures Undergoing Surgical Treatment: Meta-Analysis of Randomized Controlled Trials.
Clin Orthop Surg . 2025 Feb;17(1):16-28. doi: 10.4055/cios24184.Synopsis
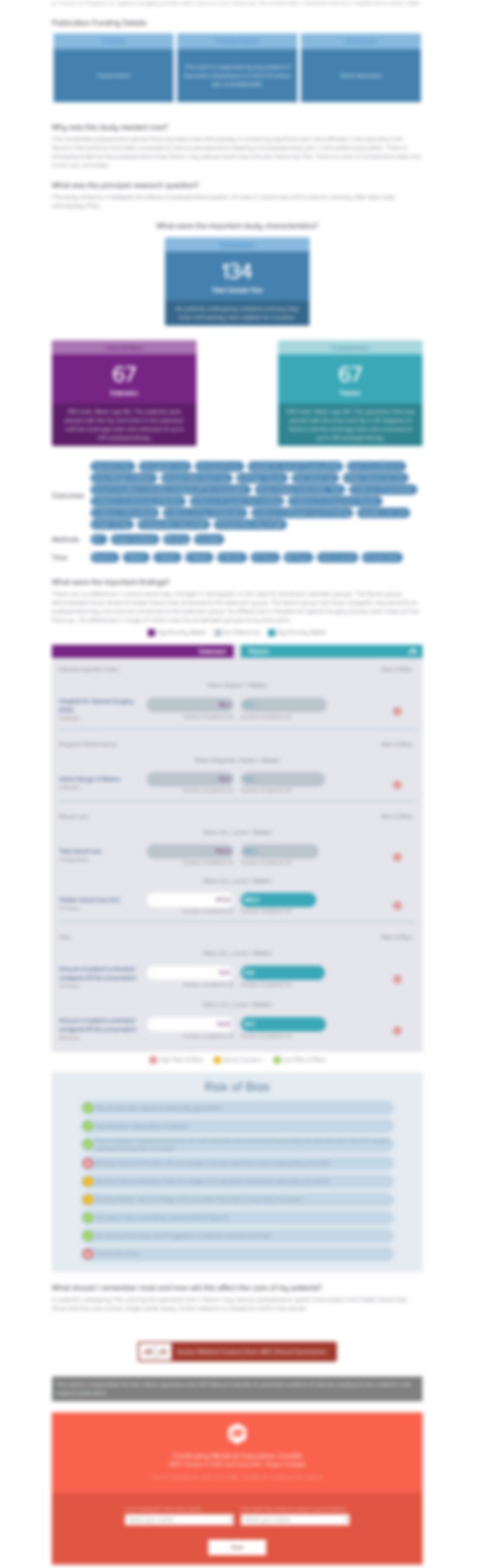
Nine randomized controlled trials including 1,024 elderly patients with hip fractures were included in this systematic review and meta-analysis comparing topical tranexamic acid (TXA) with intravenous TXA or placebo. Pooled outcomes of interest included transfusion rates, transfused blood units, hemoglobin drop, hematocrit loss, total blood loss, drain output, hospital stay, surgery duration, deep...
To view the full content, login to your account,
or start your 30-day FREE Trial today.
FREE TRIAL
LOGIN
Forgot Password?
Explore some of our unlocked ACE Reports below!
Learn about our AI Driven
High Impact Search Feature
Our AI driven High Impact metric calculates the impact an article will have by considering both the publishing journal and the content of the article itself. Built using the latest advances in natural language processing, OE High Impact predicts an article’s future number of citations better than impact factor alone.
Continue



 LOGIN
LOGIN

Join the Conversation
Please Login or Join to leave comments.