
AAOS 2025: Non-opioid Pain Protocol Provides Equivalent Relief After Shoulder Arthroplasty

AAOS 2025: Non-opioid Pain Protocol Provides Equivalent Relief After Shoulder Arthroplasty
Did you know you're eligible to earn 0.5 CME credits for reading this report? Click Here
CONFERENCE ACE REPORTS
This ACE Report is a summary of a conference presentation or abstract. The information provided has limited the ability to provide an accurate assessment of the risk of bias or the overall quality. Please interpret the results with caution as trials may be in progress and select results may have been presented.
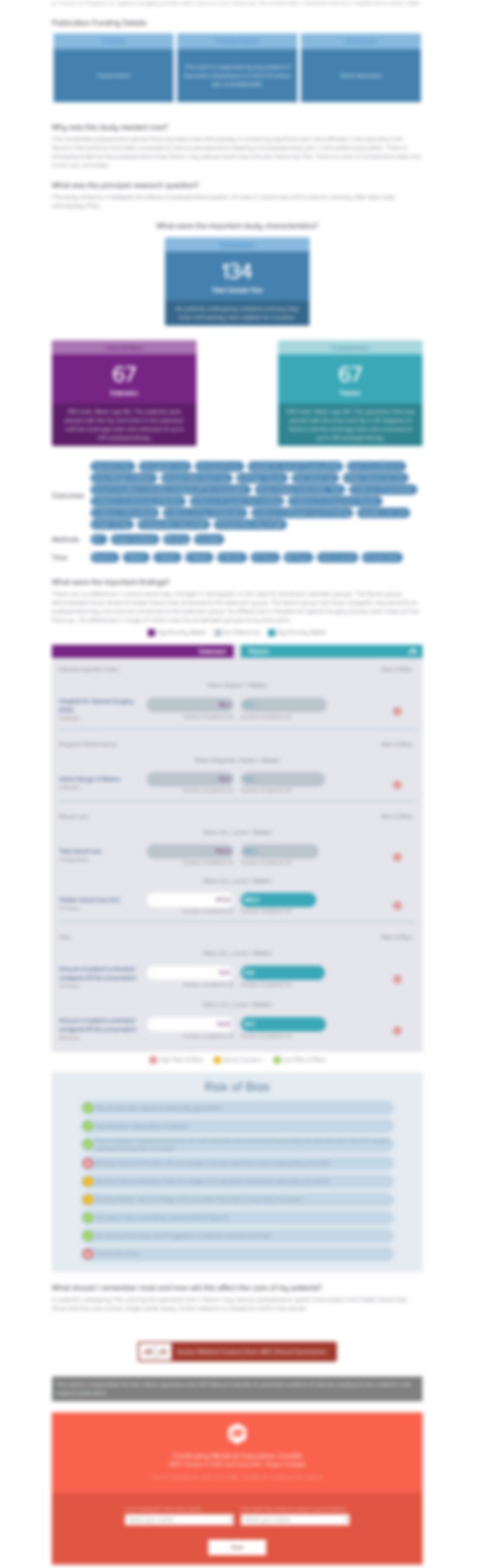
Synopsis
Seventy-four patients undergoing anatomic or reverse total shoulder arthroplasty were randomized to receive either a non-opioid multimodal pain protocol (n=37) or a multimodal protocol with 28 tablets of 5 mg oxycodone (n=37). The primary outcome of interest was opioid utilization (measured in morphine milligram equivalents). Secondary outcomes of interest included visual analog scale (VAS) pain s...
To view the full content, login to your account,
or start your 30-day FREE Trial today.
FREE TRIAL
LOGIN
Forgot Password?
Explore some of our unlocked ACE Reports below!
Learn about our AI Driven
High Impact Search Feature
Our AI driven High Impact metric calculates the impact an article will have by considering both the publishing journal and the content of the article itself. Built using the latest advances in natural language processing, OE High Impact predicts an article’s future number of citations better than impact factor alone.
Continue



 LOGIN
LOGIN

Join the Conversation
Please Login or Join to leave comments.