
Esketamine with Hip Nerve Block Provides Effective Sedation And Analgesia In Elderly THA Patients

Esketamine with Hip Nerve Block Provides Effective Sedation And Analgesia In Elderly THA Patients
A subanesthetic dose of esketamine combined with hip peripheral nerve block has good sedative and analgesic effects in elderly patients undergoing total hip arthroplasty: A randomized-controlled trial.
Jt Dis Relat Surg . 2023 Sep 16;34(3):548-556.Synopsis
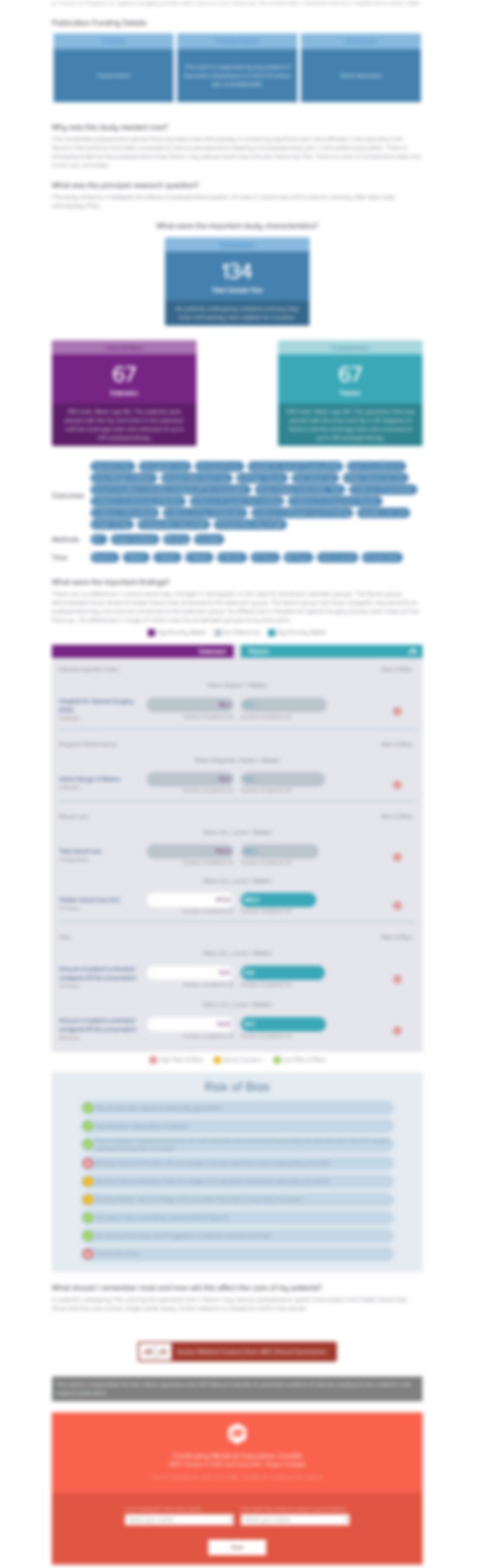
One hundred twenty elderly patients undergoing total hip arthroplasty were randomized to receive a subanesthetic dose of esketamine combined with hip capsule peripheral nerve block (n=40)(Group A), esketamine with lumbar plexus block (n=40)(Group B), or esketamine for general anesthesia (n=40)(Group C). The primary outcomes of interest were onset time of anesthesia, duration of block, postoperativ...
To view the full content, login to your account,
or start your 30-day FREE Trial today.
FREE TRIAL
LOGIN
Forgot Password?
Explore some of our unlocked ACE Reports below!
Learn about our AI Driven
High Impact Search Feature
Our AI driven High Impact metric calculates the impact an article will have by considering both the publishing journal and the content of the article itself. Built using the latest advances in natural language processing, OE High Impact predicts an article’s future number of citations better than impact factor alone.
Continue



 LOGIN
LOGIN

Join the Conversation
Please Login or Join to leave comments.