
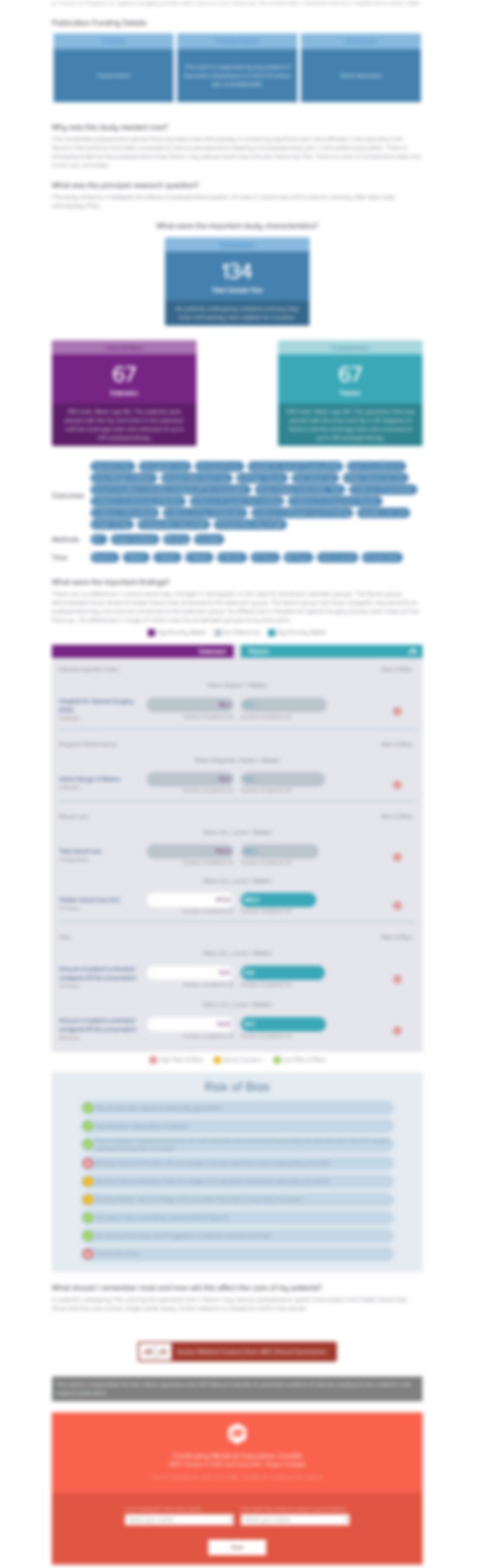
Weak evidence suggests Botulinum Toxin Type A may improve some outcomes in cerebral palsy

Weak evidence suggests Botulinum Toxin Type A may improve some outcomes in cerebral palsy
Botulinum toxin type A in the treatment of lower limb spasticity in children with cerebral palsy.
Cochrane Database Syst Rev. 2019. DOI: 10.1002/14651858.CD001408.pub2Did you know you're eligible to earn 0.5 CME credits for reading this report? Click Here
Synopsis
Cerebral palsy is a common childhood condition, with spasticity being one of its most disabling complications. Botulinum Toxin has long been used as first line treatment. The authors performed a Cochrane review of all randomized controlled trials comparing Botulinum Toxin to placebo or an active comparator. Overall, 31 trials randomized 1508 patients. The overall quality of the evidence was low or...
To view the full content, login to your account,
or start your 30-day FREE Trial today.
FREE TRIAL
LOGIN
Forgot Password?
Explore some of our unlocked ACE Reports below!
Learn about our AI Driven
High Impact Search Feature
Our AI driven High Impact metric calculates the impact an article will have by considering both the publishing journal and the content of the article itself. Built using the latest advances in natural language processing, OE High Impact predicts an article’s future number of citations better than impact factor alone.
Continue



 LOGIN
LOGIN

Join the Conversation
Please Login or Join to leave comments.