
Two-year clinicoradiographic results of biodegradable V titanium nail for pediatric forearm fracture

Two-year clinicoradiographic results of biodegradable V titanium nail for pediatric forearm fracture
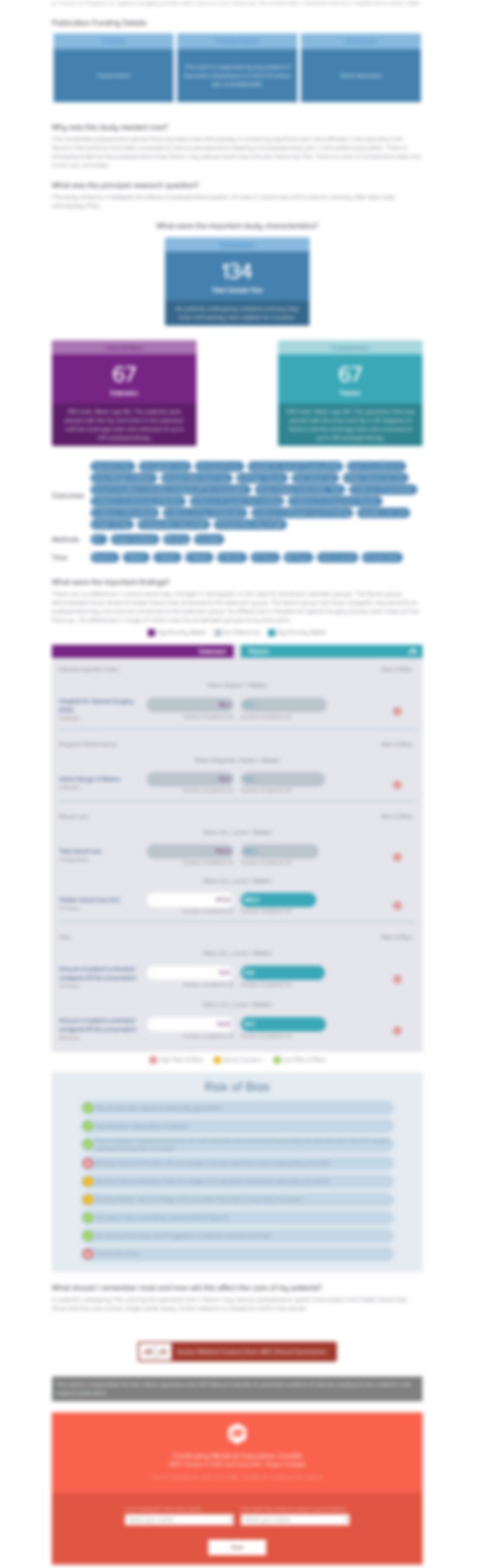
Intramedullary nailing of forearm shaft fractures by biodegradable compared with titanium nails: Results of a prospective randomized trial in children with at least two years of follow-up
Biomaterials. 2018 Dec;185:383-392. doi: 10.1016/j.biomaterials.2018.09.011Synopsis
35 pediatric patients with a closed, diaphyseal forearm fracture were randomized to fixation with either biodegradable intramedullary nailing (BIN) or titanium elastic static intramedullary nailing (ESIN). Patients were assessed after 2 years for results related to range of motion in forearm rotation, wrist elbow flexion-extension, wrist flexion-extension, fracture healing, radial and ulnar angula...
To view the full content, login to your account,
or start your 30-day FREE Trial today.
FREE TRIAL
LOGIN
Forgot Password?
Explore some of our unlocked ACE Reports below!
Learn about our AI Driven
High Impact Search Feature
Our AI driven High Impact metric calculates the impact an article will have by considering both the publishing journal and the content of the article itself. Built using the latest advances in natural language processing, OE High Impact predicts an article’s future number of citations better than impact factor alone.
Continue



 LOGIN
LOGIN

Join the Conversation
Please Login or Join to leave comments.