
AAOS2019: Intraoperative bupivicaine superior to placebo for reduction of pain in knee arthroscopy
CONFERENCE ACE REPORTS
This ACE Report is a summary of a conference presentation or abstract. The information provided has limited the ability to provide an accurate assessment of the risk of bias or the overall quality. Please interpret the results with caution as trials may be in progress and select results may have been presented.
Synopsis
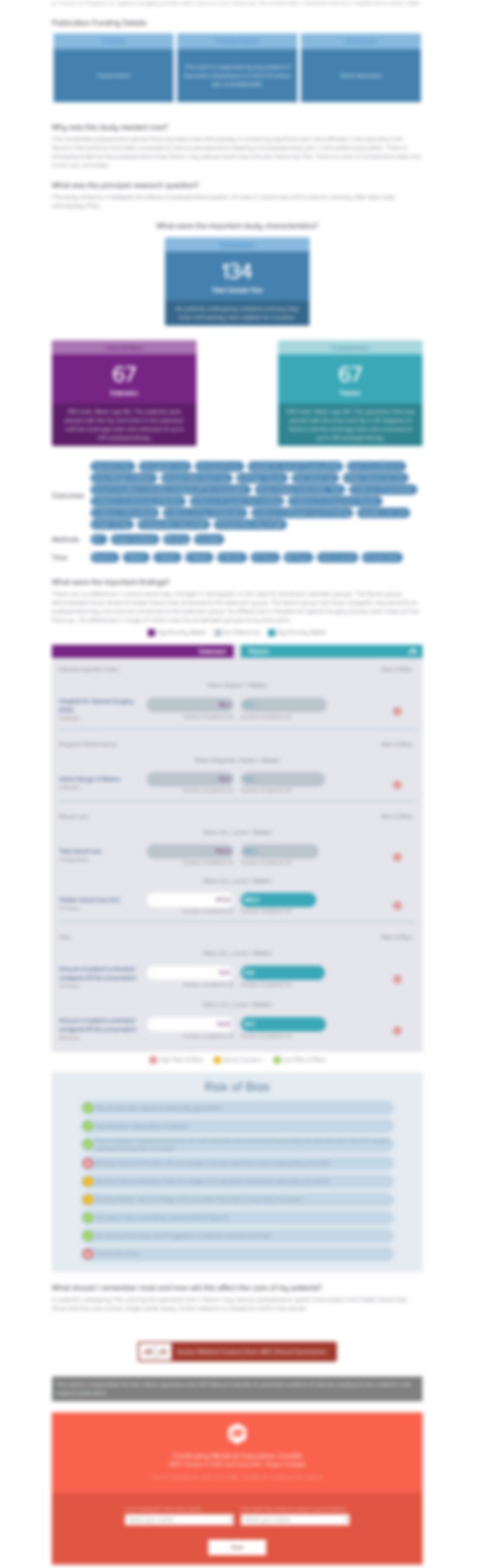
277 patients scheduled for knee arthroscopy were randomized analgesia via portal injection with either 20mL 0.5% bupivacaine, 20mL 0.25% bupivacaine, or 20mL normal saline. Follow up was conducted up to 2 weeks postoperatively. The primary outcomes of interest were pain (measured with VAS) and narcotic consumption. The result from this study found the high dose bupivacaine treatment to result in s...
To view the full content, login to your account,
or start your 30-day FREE Trial today.
FREE TRIAL
LOGIN
Forgot Password?
Explore some of our unlocked ACE Reports below!
Learn about our AI Driven
High Impact Search Feature
Our AI driven High Impact metric calculates the impact an article will have by considering both the publishing journal and the content of the article itself. Built using the latest advances in natural language processing, OE High Impact predicts an article’s future number of citations better than impact factor alone.
Continue



 LOGIN
LOGIN

Join the Conversation
Please Login or Join to leave comments.