
Condoliase Improves Quality of Life in Lumbar Disc Herniation

Condoliase Improves Quality of Life in Lumbar Disc Herniation
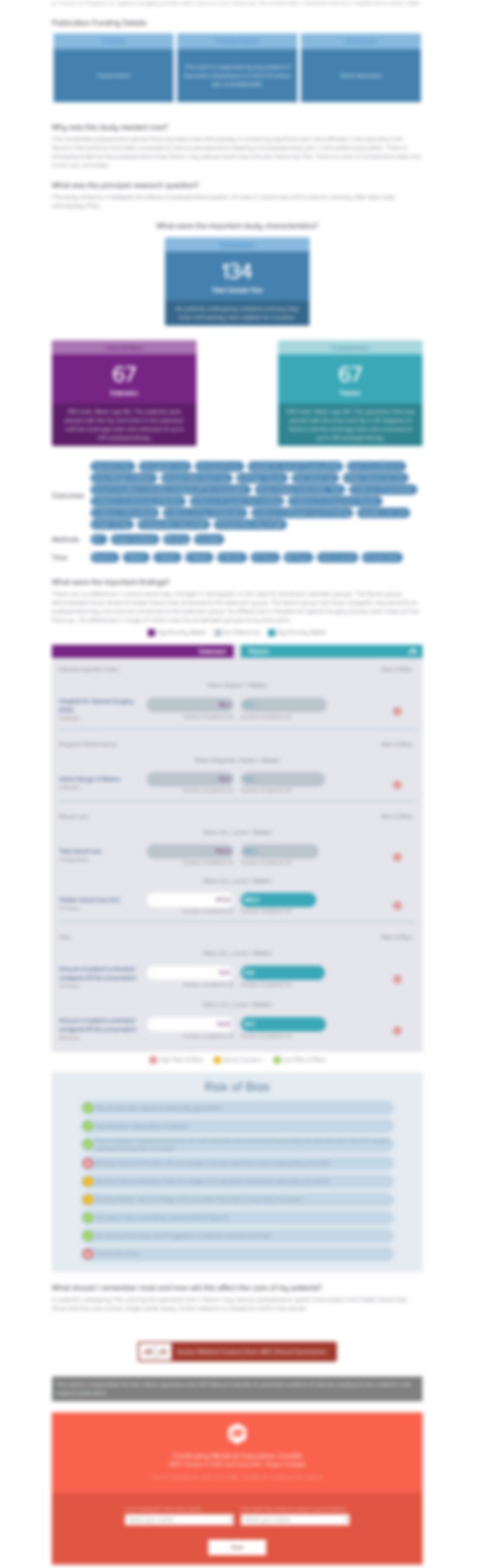
Impact of Condoliase on Health-related Quality of Life in Participants With Radicular Leg Pain Associated With Lumbar Disk Herniation: Results From a United States Phase 3 Clinical Trial.
Spine (Phila Pa 1976) . 2025 Jun 15;50(12):796-803.Synopsis
Three hundred forty-one randomized patients with Lumbar Disk Herniation (LDH) -associated unilateral radicular leg pain were analyzed (modified ITT): condoliase (n=169) vs sham (n=172). The primary outcomes for this exploratory report were HRQoL changes (EQ-5D-5L domains and EQ-VAS) at weeks 13 and 52. Secondary outcomes included SF-36 PCS/MCS, PGIC and CGIC responder proportions, and WPAI domains...
To view the full content, login to your account,
or start your 30-day FREE Trial today.
FREE TRIAL
LOGIN
Forgot Password?
Explore some of our unlocked ACE Reports below!
Learn about our AI Driven
High Impact Search Feature
Our AI driven High Impact metric calculates the impact an article will have by considering both the publishing journal and the content of the article itself. Built using the latest advances in natural language processing, OE High Impact predicts an article’s future number of citations better than impact factor alone.
Continue



 LOGIN
LOGIN

Join the Conversation
Please Login or Join to leave comments.