
Nabiximol effective and safe in managing spasticity in patients with refractory MS

Nabiximol effective and safe in managing spasticity in patients with refractory MS
A double-blind, randomized, placebo-controlled, parallel-group study of Sativex, in subjects with symptoms of spasticity due to multiple sclerosis
Neurol Res. 2010 Jun;32(5):451-9. doi: 10.1179/016164109X12590518685660Synopsis
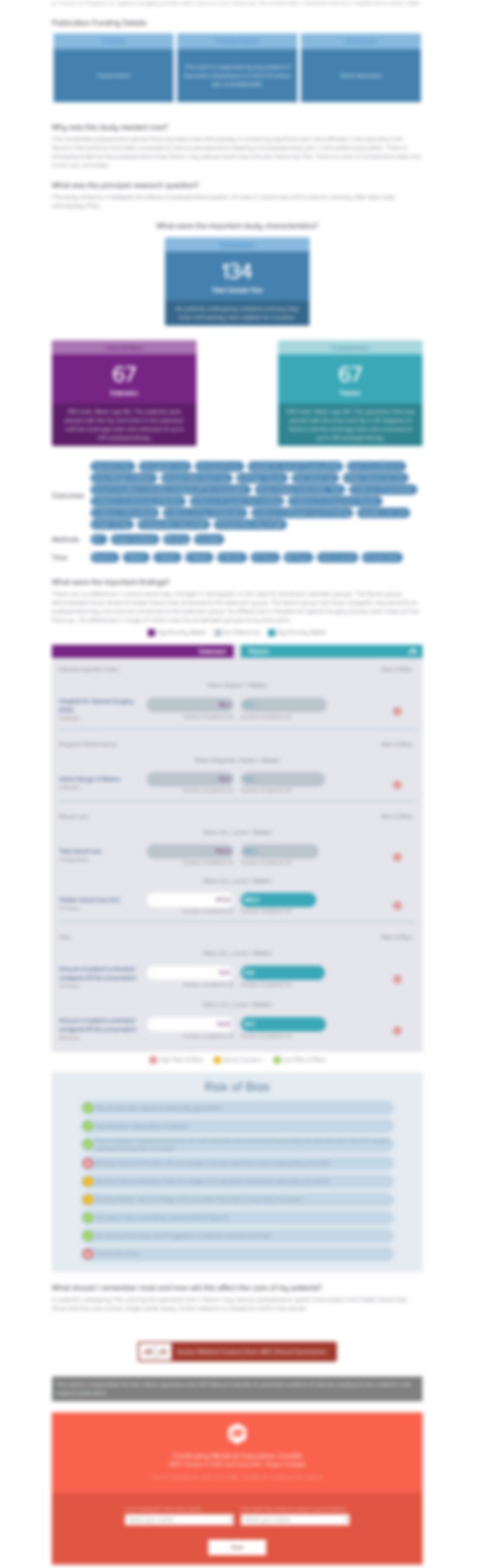
337 patients diagnosed with multiple sclerosis (MS) and inadequately managed by current medications were randomly assigned to receive either nabiximols, a mixture of delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD), or placebo through oromucosal spray for 14 weeks. Patients were assessed on mean change in spasticity from baseline measured by the 0-10 Numerical Rating Scale (NRS), the propo...
To view the full content, login to your account,
or start your 30-day FREE Trial today.
FREE TRIAL
LOGIN
Forgot Password?
Explore some of our unlocked ACE Reports below!
Learn about our AI Driven
High Impact Search Feature
Our AI driven High Impact metric calculates the impact an article will have by considering both the publishing journal and the content of the article itself. Built using the latest advances in natural language processing, OE High Impact predicts an article’s future number of citations better than impact factor alone.
Continue



 LOGIN
LOGIN

Join the Conversation
Please Login or Join to leave comments.