
DATA IS THE NEW OIL: OrthoEvidence Amasses a One of a Kind Analyzable Database (Updated)

DATA IS THE NEW OIL: OrthoEvidence Amasses a One of a Kind Analyzable Database (Updated)
Vol: | Issue: | Number: | ISSN#: 2563-5972
November 29, 2022 | Article No. 87

DATA IS THE NEW OIL: OrthoEvidence Amasses a One of a Kind Analyzable Database (Updated)
November 29, 2022 | Article No. 121
Mohit Bhandari MD, PhD
Steve Phillips MSc

Like oil, data is valuable, but if unrefined it cannot really be used. It has to be changed into gas, plastic, chemicals, etc. to create a valuable entity that drives profitable activity; so, must data be broken down, analysed for it to have value.
Michael Palmer
Our readers have probably become familiar with seeing the term OE M.I.N.D., whether it’s referenced in OrthoPods or in our growing line of OE Originals. In this OE Insight, we will take a dive into the inner workings of OE M.I.N.D: what it is, how it is developed, and how it is powering some of the most compelling products at OE. This article will focus on OE M.I.N.D. as well as the platform of tools in which it can be analyzed, OE Analytics.
As of November 29th, 2022, OE M.I.N.D. has over 144 million data points from more than 10,000 orthopaedic RCTs and meta-analyses consisting of 10.2 million patients. OE M.I.N.D. evaluates over 3,000 interventions used to treat nearly 200 conditions, and contains more than 20,000 outcome measures. These treatments comprise over 2,000 products from 1,000 manufacturers. In addition to clinical trial data, OE M.I.N.D. has over 30 million network insight data points, such as user views and poll results, which provide insight into the most popular topics on OE. Data is new oil in the 21st century. With a one-of-a-kind analyzable database of the best available evidence (that is, randomized clinical trials), opportunities for health care applications, decision-making, and predictive analytics remain ‘unprecedented’.
.png)
What is OE M.I.N.D.
OE M.I.N.D. (Machine learning INsights Database) is a database of the highest quality orthopaedic and physical therapy research consisting of randomized controlled trials (RCTs) and meta-analyses. Every ACE Report that is active on OE exists in the OE M.I.N.D. database, and since the development of ACE Report 2.0, these entries are used to generate in depth graphs for the ACE Reports.

The goodness of the data representation has a large impact on the performance of machine learners on the data: a poor data representation is likely to reduce the performance of ecan an advanced, complex machine learner...
Maryam M Najafabadi, Journal of Big Data
Quality Control: Structured Dataset Development
Quality control is a critical focus of the database. The pitfalls of large administrative databases and similar big datasets that hinders machine learning applications has largely been a lack of unstructured datasets. At OrthoEvidence, we have taken important steps to create the largest structured database of orthopaedic evidence in the world.
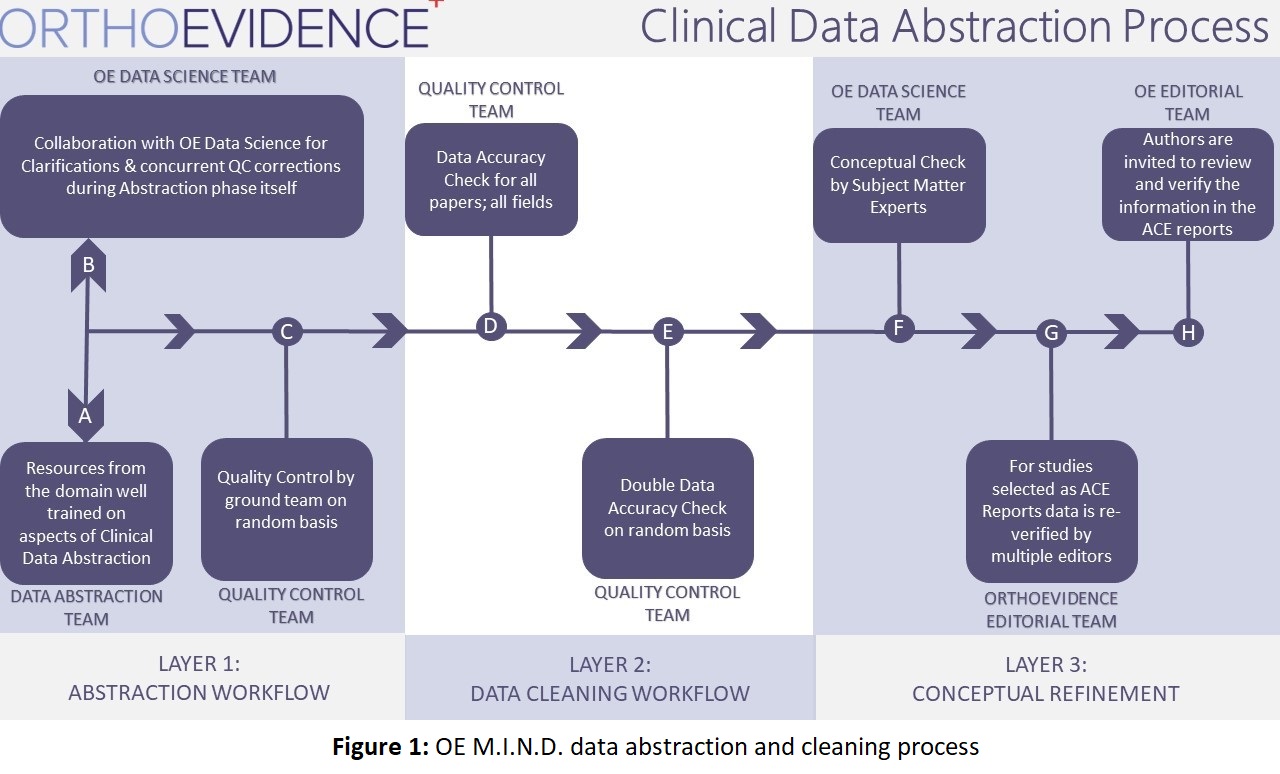
Every month a systematic search of Pubmed is performed for all high-quality orthopaedic publications. These publications are then screened for inclusion on OE based on study topic and study quality. Once screened, these articles are tagged and extracted in full, including anatomical region, conditions, treatments, and all data relating to timepoints, demographics and outcomes. Once extracted, each study receives a full cleaning, with one team verifying data accuracy and another cleaning concepts such as treatment names, outcome names, and other orthopaedic related concepts (Figure 1).
OE Analytics: The New Wave of Predictive Analytics
A large database of over 144 million datapoints requires a suite of tools to harness its potential. We will soon launch OE Analytics (beta) as a suite of 7 tools, each with a different function. The future of OE Analytics will continue to evolve as new tools are added, creating new data analysis possibilities within OE M.I.N.D.. The use of machine learning is being developed in several tools, which allow automation of analysis within our system, and automatic detection of trends in evidence of significance and interest.
What analysis can be performed?
The seven flagship tools for OE Analytics provide in depth analysis using a variety of different processes.

What used to take us months, now takes minutes. Our powerful automated meta-analyzer can perform meta-analysis from our dataset in a push of a few buttons. The power to perform hundreds of meta-analyses has arrived.
Mohit Bhandari, Editor in Chief
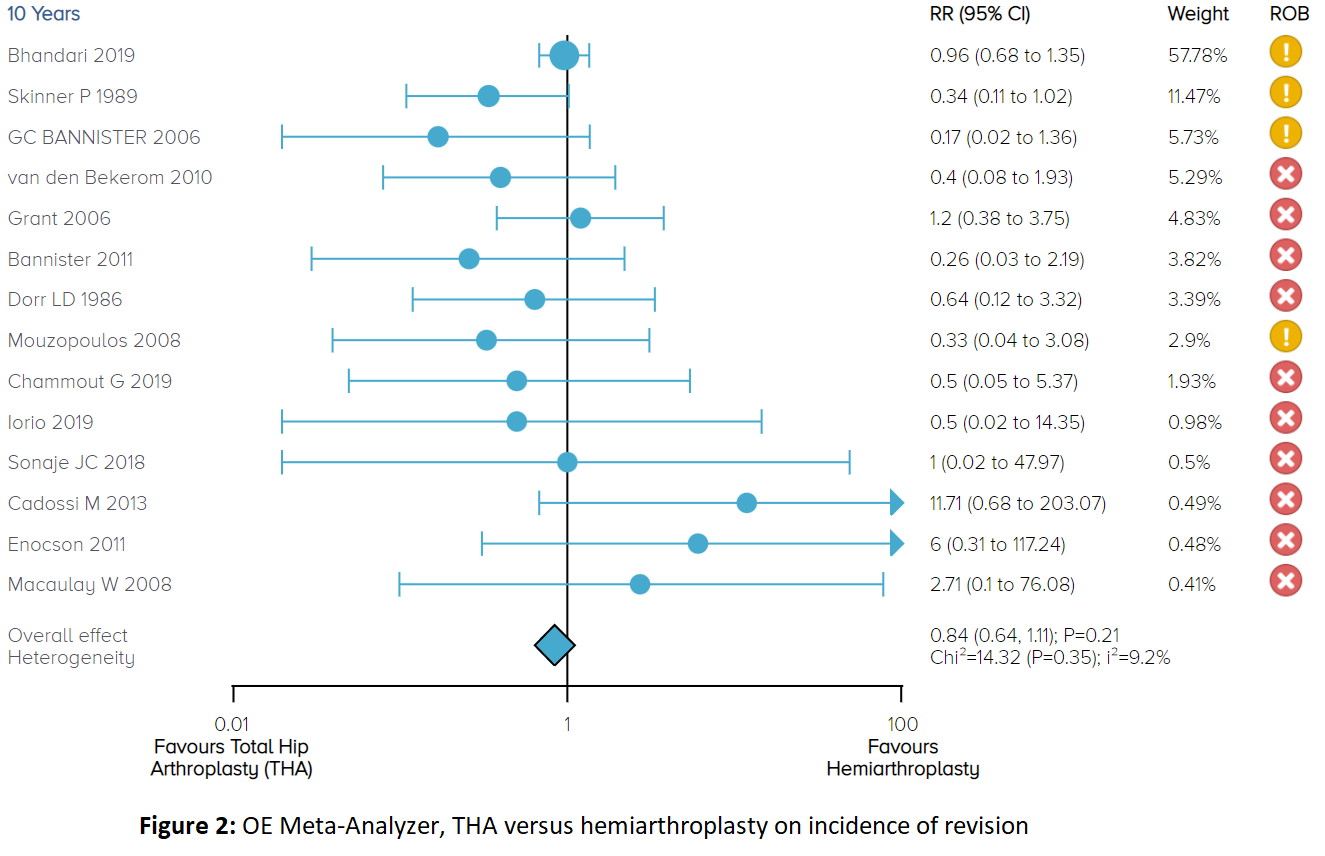
OE Meta Analyzer Tool: Analyses in Minutes (Not Months)
Meta-analysis is a powerful tool for combining the results of prior research in the pursuit of health care decisions. A methodologically rigorous meta-analysis requires a comprehensive search of the literature, a critical appraisal of the quality of evidence, the inclusion of randomized trials (ideally) and appropriately statistical methodology to combine studies (when appropriate). The time consuming aspects of meta-analysis include the search, data abstraction and quality assessments. The OE Meta Analyzer tool allows you to perform a Meta-Analysis on RCTs in the OE M.I.N.D. database with the click of a few buttons. Since the OE M.I.N.D. database has already performed your literature search and data extraction, all you need to do is leverage the extensive tagging system in order to run a meta-analysis in just a couple of minutes. The OE Meta Analyzer allows comparisons of two treatments or devices directly to one another on over 20,000 efficacy and safety outcomes where there are RCTs directly comparing the two available.
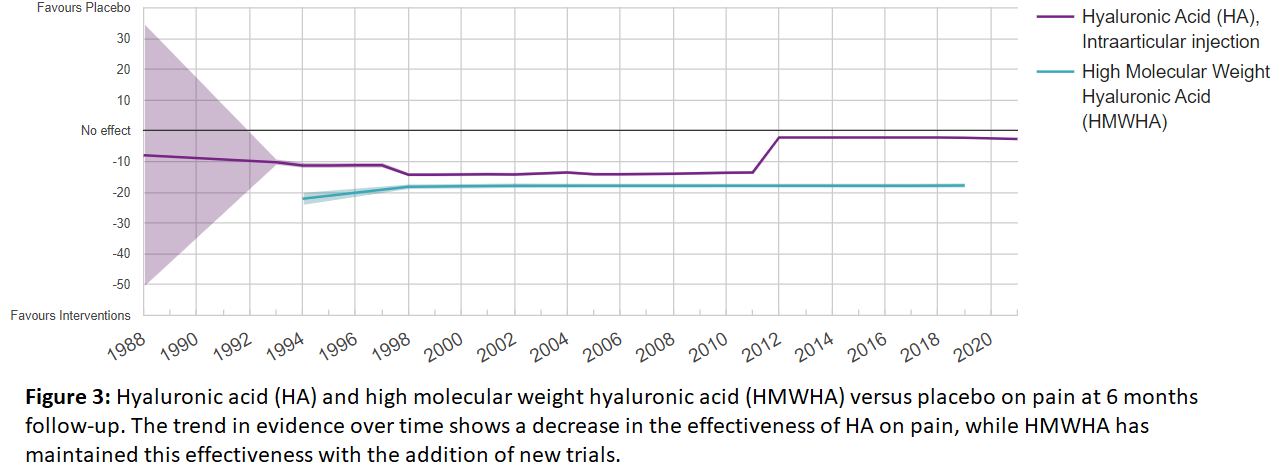
OE Forecaster Tool: Let the Past, Help Predict the Future
The OE Forecaster allows you to examine trends in evidence over time, as well as forecast the efficacy or safety of a given treatment or product compared to control or another product. The OE Forecaster enables you to perform a Cumulative Meta-Analysis on RCTs, and presents the data as a trend, with each study being added sequentially to the group. Examining these trends can inform when a treatment or product is at risk of being viewed as non-efficacious with additional evidence, or alternatively, when treatments and devices are poised to have significant perceived benefits to patient outcomes with additional studies. As with the OE Meta-Analyzer, these analyses can be achieved quickly due to our data extraction and tagging system.
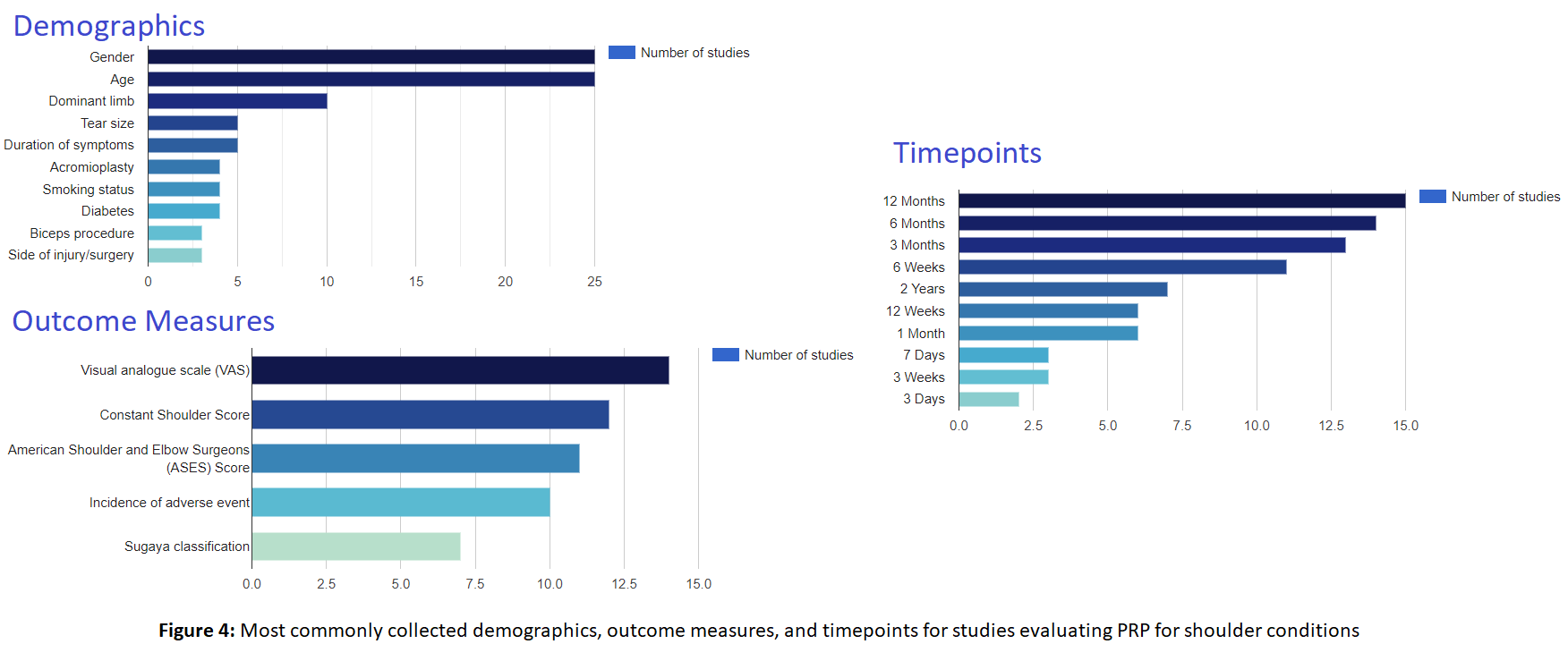
OE Research Planning Tool
The OE Research Planning Tool eases the protocol development process, by utilizing data from thousands of randomized controlled trials to help inform your study design. Saving time and effort on literature review or the use of advisory boards, the Research Planning Tool provides unrivaled insights into the most appropriate variables for your study. Using the OE Research Planning Tool, you can determine the optimal comparators, demographics, outcome measures, follow-ups, and sample size for your trial. The OE Research Planning Tool will allow you to make informed decisions in your study design process by providing the most relevant components for any comparison you wish to make.
OE Regulatory Planning Tool
Whether preparing for a regulatory submission or performing a literature search, the OE Regulatory Planning Tool gives access to thousands of Randomized Controlled Trials and Meta- Analyses to help you scope the landscape of evidence. This tool will allow you to effortlessly filter, sort and search for studies and outcomes related to a given intervention, product, or comparison. The OE Regulatory Planning Tool provides an overview of geographical location, demographics, inclusion/exclusion criteria, follow-up points, and outcome measures in order to determine the breadth of research on a topic of interest.
OE Market Analysis
Whoever holds the most evidence can hold the narrative in a field. The OE Market Analysis Tool provides an opportunity to explore what companies have the largest number of high-quality research evidence (that is, evidence from randomized trials) in a given field of study. The analysis provides the number of studies and cumulative number of patients over time per company, and includes our OE Power Ranking, which ranks the companies based on their overall research impact. The Market Analysis tool also breaks down publications based on the country in which they were conducted, indicating the largest impact for studies conducted in those countries.
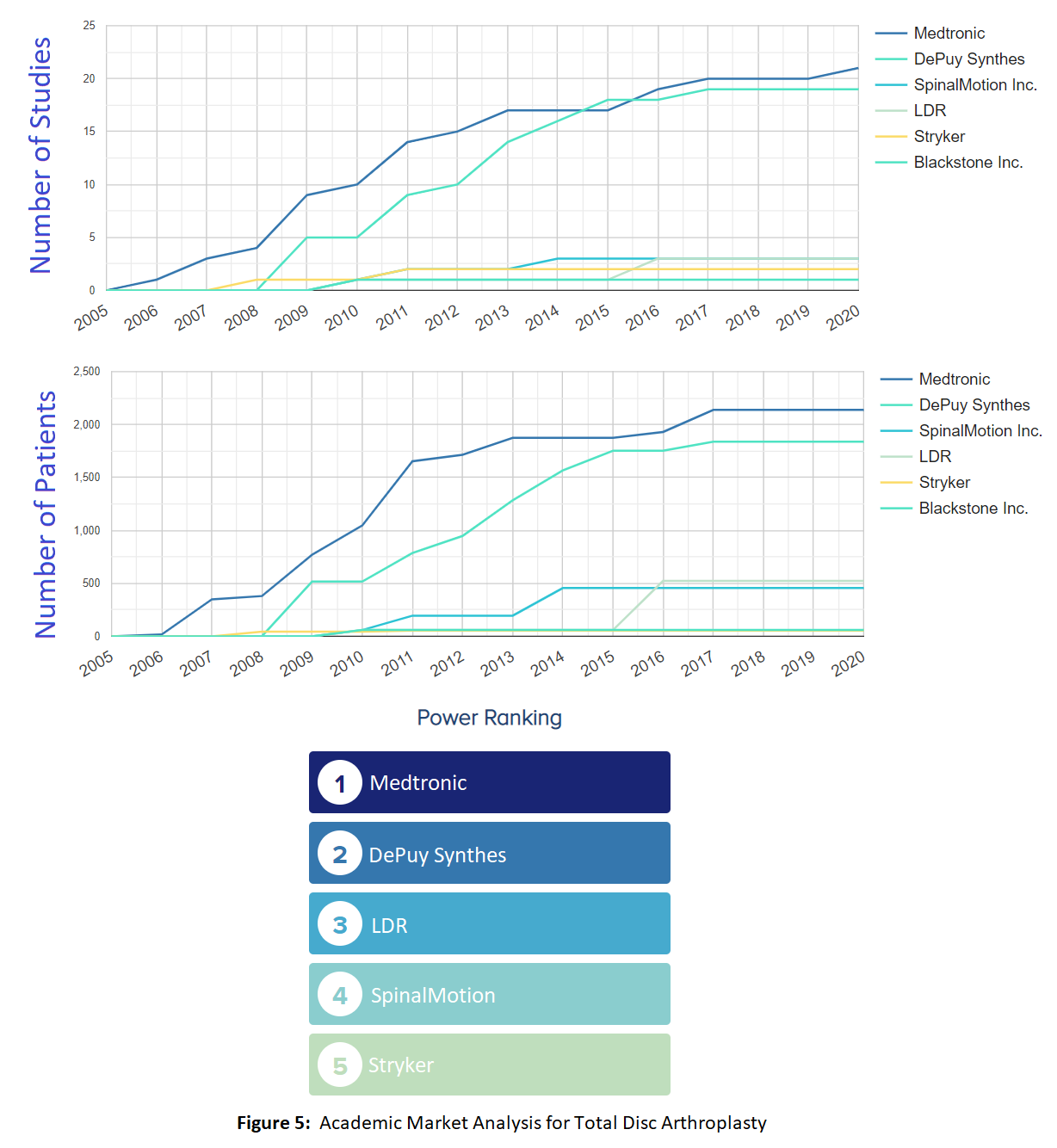
OE Ongoing Trial Report Tool
OE Ongoing Trial Reports allows you to harness the power of the clinicaltrials.gov database in order to keep up with all prospective and currently ongoing clinical trials. OE takes the entire clinicaltrials.gov database and filters out all non-orthopaedic trials, as well as all completed trials in order to provide only current and projected orthopaedic trials. OE M.I.N.D.s filtering system is then applied to all studies to allow you to quickly and effortlessly examine ongoing trials by intervention, condition, the company conducting the trial, and other fields.
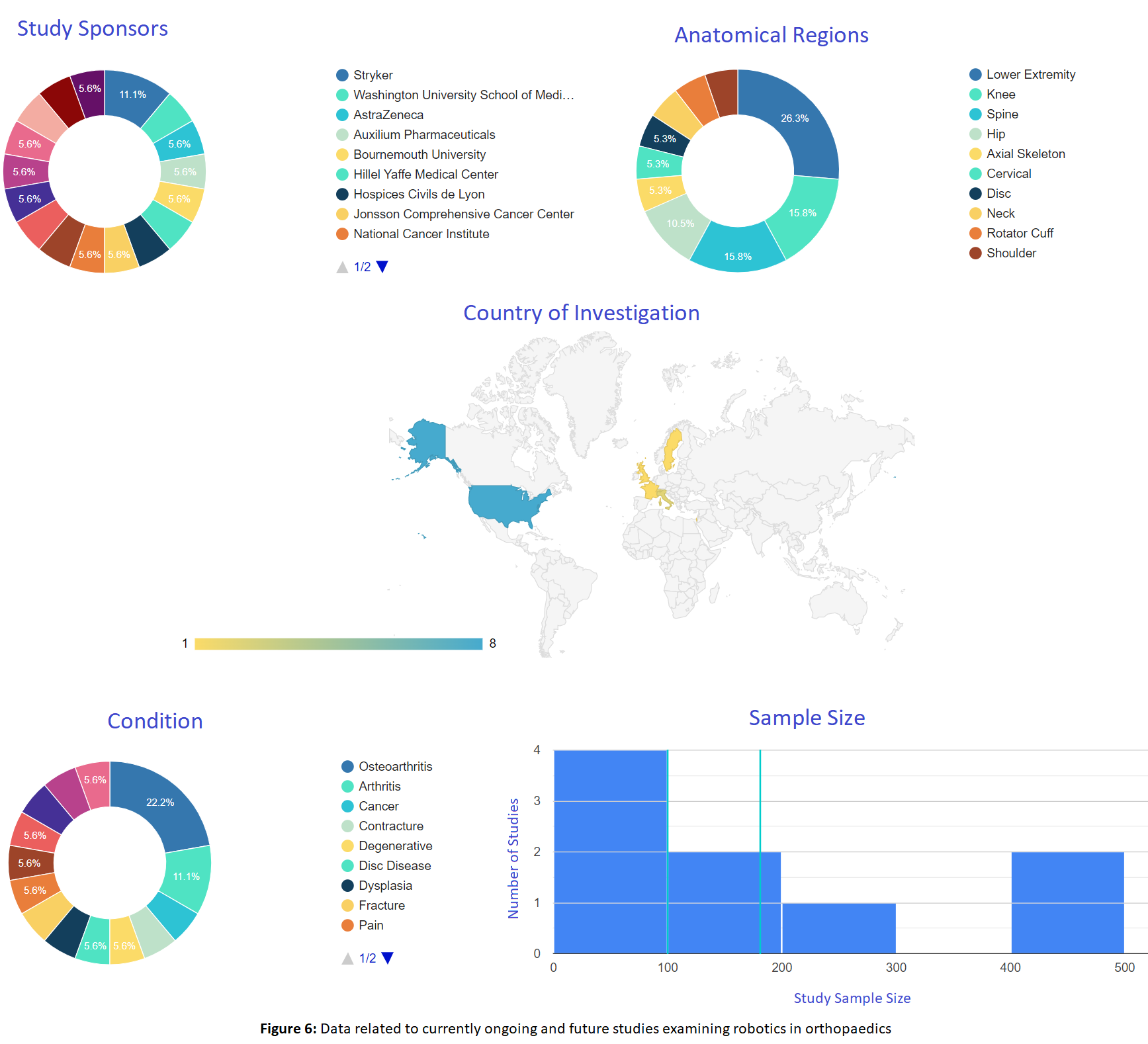
OrthoVision Tool
Providing insights into the hot and trending topics in orthopaedics, OrthoVision give you insights into what your peers are reading to ensure you don’t miss important evidence. But more importantly, OrthoEvidence’s phenotyping algorithm provides additional insights, allowing deeper insights on explorer vs expert vs enthusiast phenotypes. OrthoVision looks beyond specialty training (orthopaedic surgeon, physiotherapist, sports medicine, etc) and digs deeper into finding you insights from peers closer to your phenotype. Learn what explorers (those who lead innovation and research) are reading versus the experts, for example.
In Development
OE Analytics looks to continuously add more tools to its platform, and designs for future tools are ongoing. One example includes a sample size calculator, which uses the existing data in OE M.I.N.D. and our meta-analysis tool to automatically calculate the effect size and standard deviation for the sample size calculation thus removing the need for a literature search and manual calculations for a sample size, simply set your significance and power and get your sample size. An additional tool looks to match an author’s paper with the best fit journal based on data from over 10,000 papers in OE M.I.N.D. in order to optimize the submission process. Further tools will look to foster research networks amongst our users, helping to identify sites for multicentre research projects.
Contributors
© OrthoEvidence Inc. All Rights Reserved.




 LOGIN
LOGIN


