HIGHLIGHTS
- - OE M.I.N.D. contains data from 739 RCTs related to shoulder conditions with nearly 120,000 patients, and is a powerful and efficient tool to auto generate evidence reports regarding a clinical topic.
- - Patients with rotator cuff tendinopathy who received a single or two doses of platelet-rich plasma (PRP) injection demonstrated superior outcomes in composite clinical outcomes compared with those who received placebo or dry needling in follow-up longer than 3 weeks.
- - PRP injection demonstrated superior outcomes in pain compared with those who received placebo or dry needling in follow-up duration of more than 3 weeks to 6 months. There was no significant difference between PRP injection and control in pain within 3 weeks or in 6 to 12 months of follow-up.
- - Injection of PRP appears to be safe for treatment of rotator cuff tendinopathy. No serious adverse events were reported post treatment.
- - There are 2 studies aiming to recruit 40 patients currently ongoing studies that investigates the effects of PRP in treating rotator cuff tendinopathy using data from clinicaltrials.gov. There are 35 studies aiming to recruit 1301 patients currently ongoing for an intervention of tendinopathy in the shoulder.
- - In most published studies related to PRP and shoulder, the variable of visual analogue scale for pain and a follow-up of 12 months are reported.
- - Since 2009, the manufacturers that have published the most research associated with PRP are Zimmer-Biomet, Arthrex, Regen Lab, BTI Biotechnology Institute and Arya Mabna Tashkis Corporation.
Rotator cuff tendinopathy is a chronic degenerative condition and is associated with excessive use of rotator cuff tendons and muscles or a tear (Lewis, 2009). Rotator cuff tendinopathy affects about 20% of adults and is a common cause of pain and weakness in the shoulder (Bury et al., 2018; Yamamoto et al., 2010). The recommended conservetive treatments include nonsteroidal anti-inflammatory drugs (NSAIDs), patient education, exercise and physical therapy (Bury et al., 2018; Diercks et al., 2014; Tashjian, 2011).
The research on platelet-rich plasma (PRP) for musculoskeletal conditions has become popular in recent years. Autologous or allogeneic PRP is obtained by centrifuging venous blood, and contains a high concentration of platelets. Platelets are rich in growth factors which are effective in facilitating tissue repair, especially for the structures with poor vascular supplies, and in subsequent improved healing of the affected sites (Kwong et al., 2021; Lin et al., 2019; Rha et al., 2013). To ensure accuracy of injection, ultrasound-guided injection is usually performed (Kesikburun et al., 2013; Rha et al., 2013; Sari et al., 2020). In an Advanced Clinical Evidence (ACE) Review (https://myorthoevidence.com/AceReports/Report/4891), we found evidence from 10 randomized controlled trials (RCTs) that compared with control, intraoperative or immediately postoperative application of PRP provided favourable composite clinical outcomes at 12 months in patients with rotator cuff tears necessitating a surgery. In this OE Original, we present evidence from RCTs assessing the efficacy of PRP versus control in patients with rotator cuff tendinopathy including those with a partial tear and did not have a repairing surgery.
We conduct our analytics using OE M.I.N.D. that include a scoping review of published studies, meta-analysis results and quality of evidence, and a profile of ongoing trials about PRP for shoulder disease. All of the data were extracted from RCTs by experienced medical literature reviewers. OE M.I.N.D. updates data on a daily basis, with new trials and data being constantly added. The results in this OE Original were based on analyses performed on November 19,
2021.
1. OE M.I.N.D. Meta Analyzer --- Overview of the available evidence
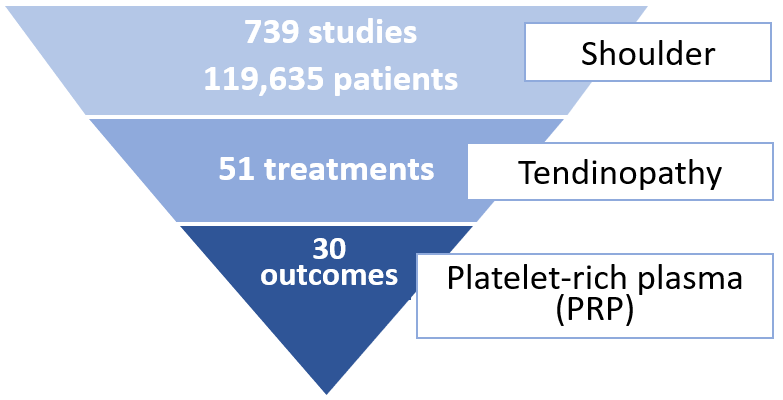
Nearly 120,000 patients across 739 studies were reported for shoulder conditions. There are 51 treatments that were studied for rotator cuff tendinopathy, and 30 outcome measures were reported at various followup durations evaluating efficacy of PRP in treating the condition (Figure 1).
Figure 1. Summary data of research topic according to anatomical region, condition and treatment
2. OE M.I.N.D. Meta Analyzer --- Effectiveness of treatments
We identified 3 RCTs investigating the effectiveness of subacromial injection of PRP compared with control for rotator cuff tendinopathy. Of them, 2 studies administered a single injection of PRP (Kesikburun et al., 2013; Sari et al., 2020) and 1 study administered two injections at 4-week intervals (Rha et al., 2013). All the studies used ultrasound in guidance of injection. The longest follow-up among the included RCTs was 12 months (Kesikburun et al., 2013). The characteristics of the RCTs included in meta-analysis are presented in Table 1.
Table 1. Characteristics of randomized controlled trials (RCTs) included in meta-analysis | |||||
Author, Year | Country | Number of patients | Patients | PRP | Comparator(s) |
Kesikburun et al., 2013 | Turkey | 40 | Chronic rotator cuff tendinopathy or partial tendon ruptures confirmed by MRI exam (pain > 3 months) | 5 mL of PRP prepared from autologous venous blood, injection into the subacromial space (center of tear gap and the at 4 sites for a partial tear, or center and 4 points surrounding area with most echogenicity changes for tendinosis) after administration of 1 mL of 1% lidocaine; Ultrasound-guided; Single injection; Followed by an exercise program for 6 weeks. | 5 mL of saline solution, same procedures as the PRP group; Ultrasound-guided; Single injection; Followed by an exercise program for 6 weeks. |
Rha et al., 2013 | South Korea | 39 | Rotator cuff tendinopathy, i.e., tendinosis or partial tear less than 1 cm (pain > 6 months; score > 5 on a 0-10 pain VAS) | 3 mL of PRP prepared from autologous venous blood, injection into lesion of the supraspinatus tendon (or around the lesion for a partial tear) after administration of <1 mL of 0.5% lidocaine; Ultrasound-guided; 2 injections at 4-week intervals; Followed by self-exercise. | Dry needling using a similar technique as the PRP group; dry needling through the lesion of the tendon; Ultrasound-guided; 2 procedures at 4-week intervals; Followed by self-exercise. |
Sari et al., 2020 | Turkey | 129 | Rotator cuff tendinosis, with or without partial ruptures grade I or bursitis (pain > 3 months) | 5 mL PRP injection into the lateral subacromial area with subacromial bursae as the needle endpoint; Ultrasound-guided; Single injection. | Placebo group: 3 mL 1% lidocaine and 2 mL saline, same procedures as the PRP group; Ultrasound-guided; Single injection. |
PRP, platelet-rich plasma; MRI, magnetic resonance imaging; VAS, visual analogue scale.
We are presenting the meta-analysis results of composite clinical outcomes and pain within five time frames of follow-up: at 3 weeks, 3 to 6 weeks, 6 weeks to 3 months, 3 to 6 months, and 6 to 12 months post intervention. We rated the quality of evidence by GRADE assessment and applied the recommended minimal clinically important difference (MCID) for the respective outcomes to assess the magnitude of effects.
2.1 Composite clinical outcomes
(0 to 100, a higher score indicates better recovery)
American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form (ASES), Shoulder Pain And Disability Index (SPADI), and Western Ontario Rotator Cuff (WORC) Index are normalized on a 0 to 100 scale to assess patient functional recovery (Velentgas et al., 2010).
The MCID of ASES score in patients with shoulder pathologies or dysfunction is found to be 6.4 points on the 0 to 100 scale (Michener et al., 2002; McClure et al., 2003).
In the current comparison of PRP injection versus control (placebo of saline injection, or dry needling) for the composite clinical outcomes at 3 weeks, a total of 208 patients from 3 studies published between 2013 and 2020 were included in the analysis. There is no significant difference between PRP injection and control, with moderate certainty of evidence (Figure 2).
During 3 to 6 weeks of follow-up, a total of 79 patients from 2 studies published in 2013 were included in analysis. The overall effect demonstrates that PRP results in a significant improvement with patients experiencing, on average, a 13.17 [95% confidence interval (CI), 10.59 to 15.75) point improvement. The effect and 95% CI exceeded the recommended MCID of 6.4 points on the 0 to 100 scale. The certainty of the evidence was rated as high (Figure 2).
During 6 weeks to 3 months of follow-up, the overall effect of 208 patients from 3 studies demonstrates that PRP results in a significant improvement with patients experiencing, on average, a 3.61 (95% CI, 1.97 to 5.25) point improvement. During 3 to 6 months of follow-up, the overall effect of the same 3 studies shows that PRP results in a significant improvement with patients experiencing, on average, a 7.63 (95% CI, 5.24 to 10.01) point improvement. The effect and 95% CI during both periods of follow-up did not exceed the MCID. The certainty of the evidence was rated as low due to imprecision and inconsistency (Figure 2).
At 12 months of follow-up, a total of 40 patients from 1 study published in 2013 were included in the analysis. The effect demonstrates that PRP results in a significant improvement with patients experiencing, on average, a 18.77 (95% CI, 1.1 to 36.45) point improvement. The 95% CI did not exceed the MCID. The certainty of the evidence was rated as moderate (Figure 2).
Figure 2. Forest plot of composite clinical outcomes on a 0-100 scale
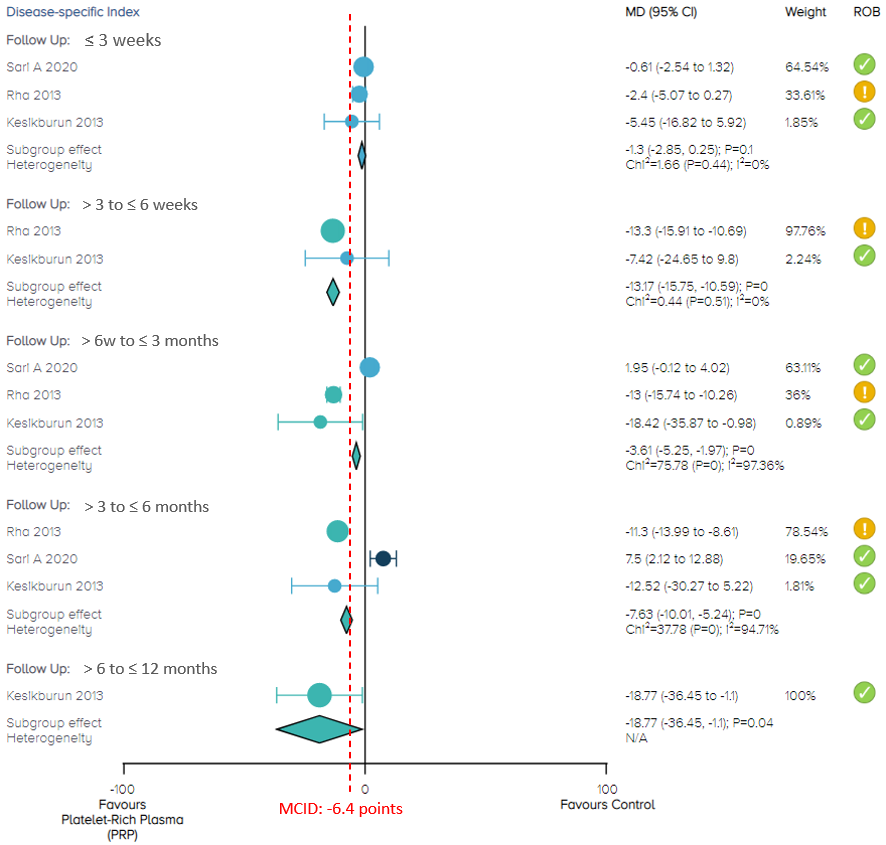
Notes: MCID = minimal clinically important difference;
ROB = risk of bias; yellow circle with an exclamation mark = have some concerns;
green circle with a check mark = low risk of bias.
2.2 Pain score (0 to 100, a higher score indicates worse pain)
Visual analogue scale (VAS) of pain, and SPADI pain subscale are normalized on a 0 to 100 scale to assess pain post intervention. The MCID of VAS pain score in patients treated for rotator cuff disease is described as 14 points on the 0 to 100 scale (Tashjian et al., 2009; Kwong et al., 2021).
In the current comparison of PRP injection versus control (placebo of saline injection, or dry needling) for pain at 3 weeks and at 12 months, there is no significant difference between PRP injection and control, with moderate certainty of evidence (Figure 3).
In the comparison of PRP injection versus control for pain, the overall effect demonstrates that PRP results in a significant reduction in pain in these three follow-up periods: 3 to 6 weeks (79 patients from 2 studies; MD, -5.26; 95% CI, -6.26 to -4.25), 6 weeks to 3 months (208 patients from 3 studies; MD, -4.84; 95% CI, -5.86 to -3.83) and 3 to 6 months (208 patients from 3 studies; MD, -4.63; 95% CI, -5.65 to -3.6). Nevertheless, these effects and 95% CIs did not exceed the MCID of 14 points on the 0 to 10 pain VAS. We rated the certainty of evidence as moderate (Figure 3).
Figure 3. Forest plot of pain on a 0-100 scale
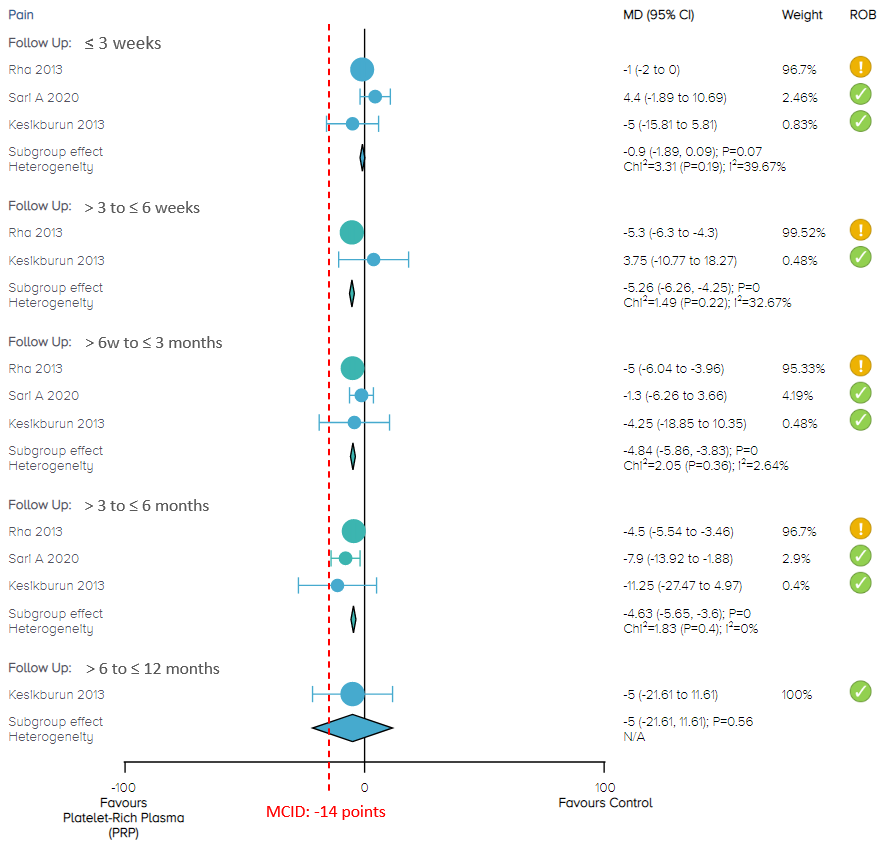
Notes: MCID = minimal clinically important difference; ROB = risk of bias.
2.3 Incidence of adverse events
No patients reported any serious treatment- related adverse events (Kesikburun et al., 2013; Rha et al., 2013).
We present a summary of the nine outcome measures at three follow-up durations in Table 2.
Table 2. Summary and certainty of the evidence
Follow-up | Composite clinical outcomes | Pain |
=< 3 weeks | No difference (Moderate certainty) | No difference (Moderate certainty) |
> 3 to 6 weeks | Favours PRP with large effect (High certainty) | Favours PRP (Moderate certainty) |
> 6 weeks to 3 months | Favours PRP (Low certainty) | Favours PRP (Moderate certainty) |
> 3 to 6 months | Favours PRP (Low certainty) | Favours PRP (Moderate certainty) |
> 6 to 12 months | Favours PRP (Low certainty) | No difference (Moderate certainty) |
PRP, platelet-rich plasma.
3. OE M.I.N.D Ongoing Trials Report
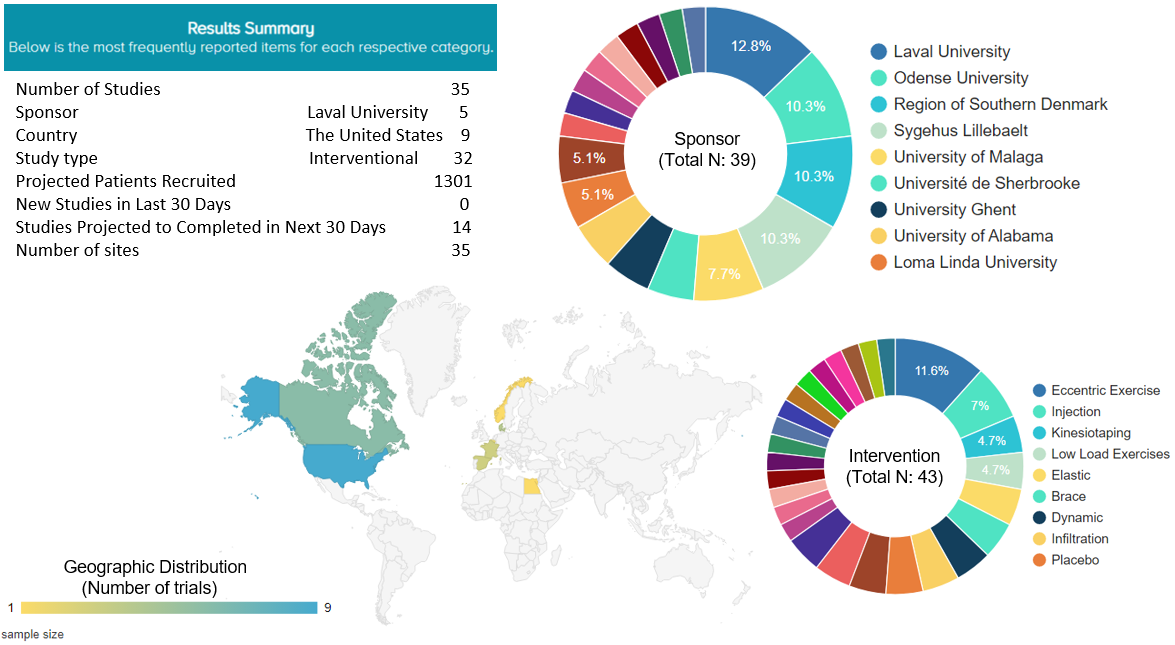
We found two registered, ongoing studies that are investigating the effects of PRP injection in treating tendinopathy in the shoulder. They are both single-center, interventional studies being conducted in the United States and aiming to recruit 40 patients.
For any treatment for tendinopathy in the shoulder, a total of 35 studies were found to be currently ongoing around the world, aiming to recruit 1301 patients. Nine of these 35 ongoing studies (25.7%) are being conducted in the United States. Five of them (11.6%) have an intervention of eccentric exercise and 3 of them (7%) have an injection as an intervention (Figure 4).
Figure 4. Ongoing trials for rotator cuff tendinopathy
4. OE M.I.N.D. Research Planning Tool
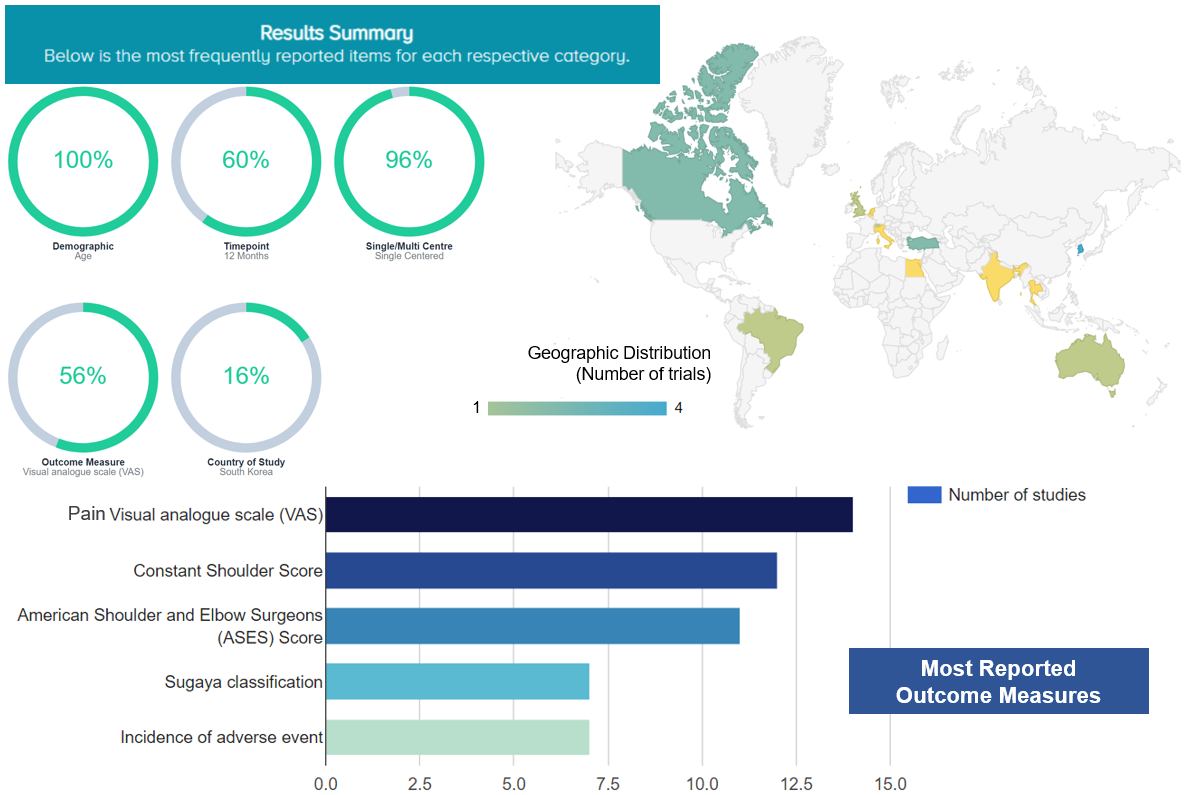
The OE M.I.N.D. Research Planning Tool provides us with an overview of characteristics of prior RCTs. For studies related to PRP and shoulder, the most frequently reported characteristics include: patient demographics, age (100% studies reported age); follow-up time point, 12 months (60% studies reported outcomes at 12 months’ follow-up); studies conducted at a single center (96%); pain VAS (56%); and the country, South Korea (16%) (Figure 5).
Figure 5. The most frequently reported characteristics of relevant studies about PRP and shoulder
5. OE MIND Academic Market Analysis --- Top Sponsors
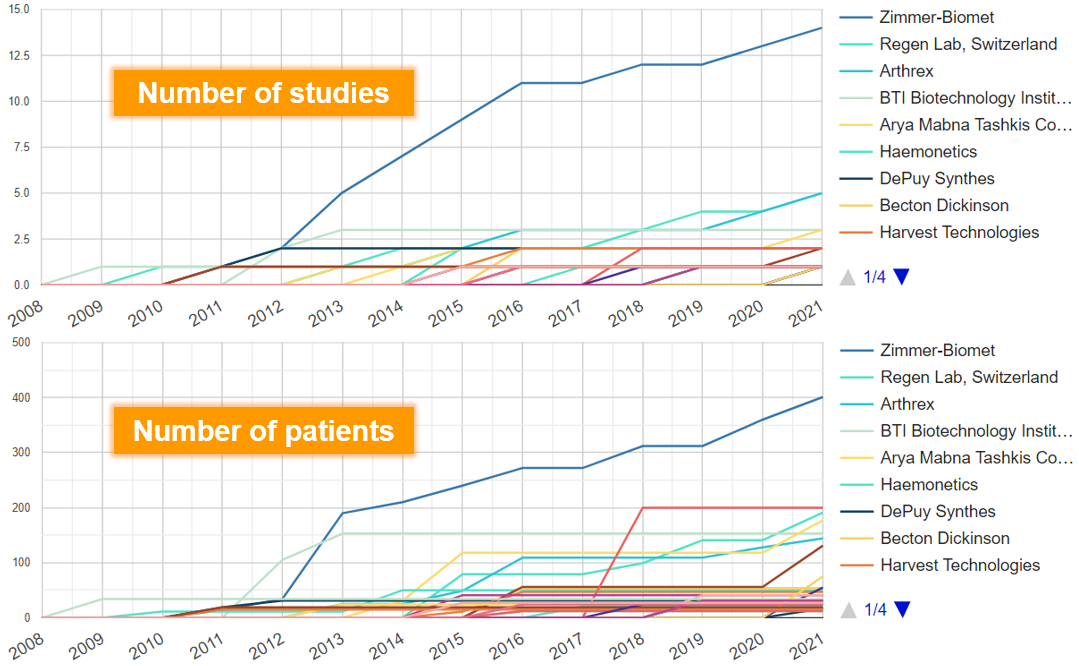
For investigating effects of PRP for any condition, we found that since 2009, the manufacturers that have published the most research are Zimmer-Biomet (N of studies=14), Arthrex (N=5), Regen Lab (N=5), BTI Biotechnology Institute (N=3) and Arya Mabna Tashkis Corporation (N=3) (Figure 6).
The manufacturers with the largest cumulative sample size are Zimmer-Biomet (N of patients=401), Sigma (N=200), Regen Lab (N=191), Arya Mabna Tashkis Corporation (N=177), BTI Biotechnology Institute (N=153) and Arthrex (N=144) (Figure 6).
Figure 6. Manufacturers with most research on PRP
Discussion
In this OE Original, we identified 3 RCTs that investigated the efficacy and safety of PRP injection versus control in patients with rotator cuff tendinopathy. Our meta-analyses showed that PRP was superior to control in composite clinical outcomes (ASES, SPADI or WORC) in medium- and long-term follow-up, i.e., longer than 3 weeks. High quality of evidence showed the efficacy greater than the MCID in composite clinical outcomes in > 3 to 6 weeks of follow-up. Moderate quality of evidence showed small benefits of PRP compared to control in pain improvement in > 3 weeks to 6 months of follow-up. No statistically significant difference was found between the two groups in pain in short-term (up to 3 weeks) or in long-term (6 to 12 months).
Previously published systematic reviews on the similar topic have shown efficacy of PRP in terms of function and pain and the outcomes are better in the longer-term than in the shorter-term (Giovannetti de Sanctis et al., 2021; Lin et al., 2019).The results in this OE Original are consistent with these findings.
No serious treatment-related adverse events were reported in the included studies. PRP is found to be a safe treatment for musculoskeletal conditions. Furthermore, PRP is a minimally invasive and relatively easy-to-administer option for patients with rotator cuff tendinopathy (Rha et al., 2013; Giovannetti de Sanctis et al., 2021).
One of the major concerns during the evidence quality assessment was the imprecision issue. We rated down one level of GRADE assessment for imprecision regarding both outcomes in several follow-up durations. Although the CIs of the outcomes excluded the no effect line, their CIs crossed the recommended MID values and clinical decisions would differ if the upper boundary versus the lower boundary of the CIs represented the true effect, for patients to achieve a minimally important improvement (Guyatt et al., 2011a). For composite clinical outcomes in two follow-up durations of > 6 weeks to 3 months, and 3 to 6 months, we also rated down one level of GRADE assessment for inconsistency (Guyatt et al., 2011b).
Additional future research with larger sample sizes, with consideration of investigating different doses of PRP, and with at least 6 months follow-up is needed to comprehensively evaluate the outcomes and associated cost, and verify the findings of the current meta-analysis results.
Bottom Line
Meta-analysis of 3 RCTs showed that for patients with rotator cuff tendinopathy, a subacromial injection of PRP is associated with benefits in composite clinical outcomes in follow-up longer than 3 weeks. Small benefits of PRP are found for pain in > 3 weeks to 6 months of follow-up. There was no significant difference between PRP and control in both outcomes in short-term (up to 3 weeks) or for pain in 6 to 12 months of follow-up. No serious adverse events were reported after treatment.
References
Diercks R, Bron C, Dorrestijn O, Meskers C, Naber R, De Ruiter T, Willems J, Winters J, Van Der Woude HJ. Guideline for diagnosis and treatment of subacromial pain syndrome: a multidisciplinary review by the Dutch Orthopaedic Association. Acta orthopaedica. 2014 Jun 1;85(3):314-22.
Giovannetti de Sanctis E, Franceschetti E, De Dona F, Palumbo A, Paciotti M, Franceschi F. The Efficacy of Injections for Partial Rotator Cuff Tears: A Systematic Review. Journal of Clinical Medicine. 2021 Jan;10(1):51.
Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, Devereaux PJ, Montori VM, Freyschuss B, Vist G, Jaeschke R. GRADE guidelines 6. Rating the quality of evidence—imprecision. Journal of clinical epidemiology. 2011 Dec 1;64(12):1283-93.
Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, Alonso-Coello P, Glasziou P, Jaeschke R, Akl EA, Norris S. GRADE guidelines: 7. Rating the quality of evidence—inconsistency. Journal of clinical epidemiology. 2011 Dec 1;64(12):1294-302.
Kesikburun S, Tan AK, Yilmaz B, Yasar E, Yazicioglu K. Platelet-rich plasma injections in the treatment of chronic rotator cuff tendinopathy: a randomized controlled trial with 1-year follow-up. The American journal of sports medicine. 2013 Nov;41(11):2609-16.
Kwong CA, Woodmass JM, Gusnowski EM, Bois AJ, Leblanc J, More KD, Lo IK. Platelet-rich plasma in patients with partial-thickness rotator cuff tears or tendinopathy leads to significantly improved short-term pain relief and function compared with corticosteroid injection: a double-blind randomized controlled trial. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2021 Feb 1;37(2):510-7.
Lin MT, Chiang CF, Wu CH, Huang YT, Tu YK, Wang TG. Comparative effectiveness of injection therapies in rotator cuff tendinopathy: a systematic review, pairwise and network meta-analysis of randomized controlled trials. Archives of physical medicine and rehabilitation. 2019 Feb 1;100(2):336-49.
Rha DW, Park GY, Kim YK, Kim MT, Lee SC. Comparison of the therapeutic effects of ultrasound-guided platelet-rich plasma injection and dry needling in rotator cuff disease: a randomized controlled trial. Clinical rehabilitation. 2013 Feb;27(2):113-22.
Sari A, Eroglu A. Comparison of ultrasound-guided platelet-rich plasma, prolotherapy, and corticosteroid injections in rotator cuff lesions. Journal of back and musculoskeletal rehabilitation. 2020 Jan 1;33(3):387-96.
Tashjian RZ. AAOS clinical practice guideline: optimizing the management of rotator cuff problems. JAAOS-Journal of the American Academy of Orthopaedic Surgeons. 2011 Jun 1;19(6):380-3.
Tashjian RZ, Deloach J, Porucznik CA, Powell AP. Minimal clinically important differences (MCID) and patient acceptable symptomatic state (PASS) for visual analog scales (VAS) measuring pain in patients treated for rotator cuff disease. Journal of shoulder and elbow surgery. 2009 Nov 1;18(6):927-32.
Velentgas P, Dreyer NA, Wu AW. Outcome Definition and Measurement. In: Velentgas P, Dreyer NA, Nourjah P, et al., editors. Developing a Protocol for Observational Comparative Effectiveness Research: A User's Guide. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013 Jan. Chapter 6. Available from: https://www.ncbi.nlm.nih.gov/books/NBK126186/




 LOGIN
LOGIN

Join the Conversation
Please Login or Join to leave comments.
Orthopaedic Surgeon - Canada
Nicotine exposure is not even mentionned once.